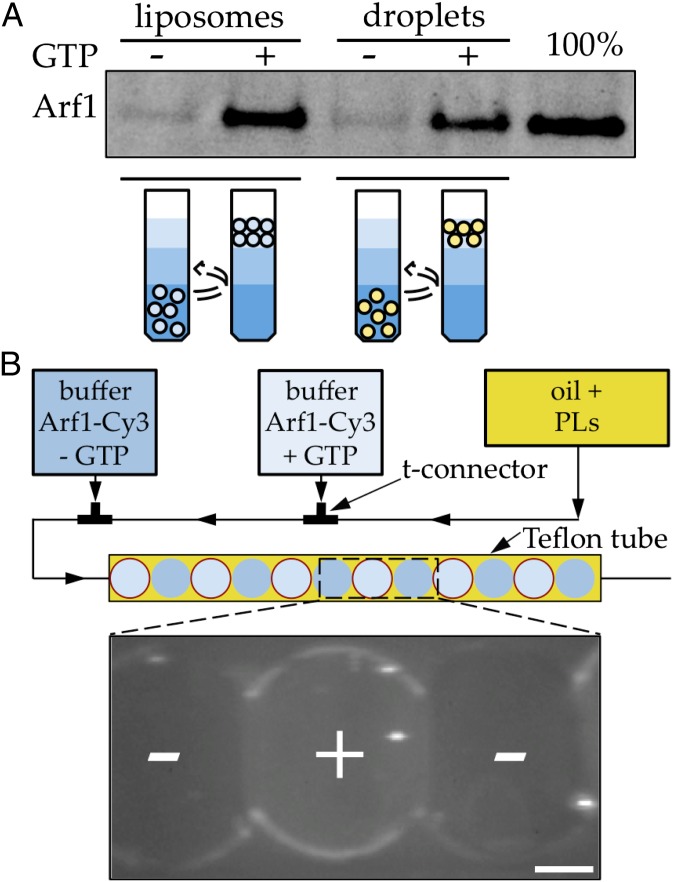
Fig. 1.
GTP-specific binding of Arf1 to LDs. (A) Arf1 binds LDs or liposomes with the same efficiency and in a GTP-dependent manner. Extruded liposomes and TAG droplets containing the same amount of exposed PL (0.5 mM) were incubated during 30 min with Arf1–GDP (500 nM) and, when indicated, with EDTA (2 mM) and GTP (100 µM) to promote activation of Arf1. After separation of the free proteins from the liposomes or TAG droplets in a sucrose gradient, the amount of Arf1 bound to the membrane was revealed by SDS-PAGE gel stained with SYPRO Orange staining. The amount of Arf1 bound to liposomes and TAG droplets is similar (lanes 2 and 4). No binding is observed in the absence of GTP (lanes 1 and 3). Lane 5 represents the amount of Arf1 input in the solutions. (B) Specific binding of Arf1 to TAG/buffer interface in buffer drops watched in epifluorescence imaging. In a microfluidics channel (Upper), micrometric buffer droplets are produced in a stream of oil containing the phospholipids. Consecutive buffer drops containing fluorescent Arf1–Cy3 (30 nM), ARNO (200 nM), and alternately GTP (50 µM) or nucleotide-free control are produced with this setup. Arf1 only labels the aqueous/TAG interface when GTP is present (center buffer drop). Limited labeling of the contour is observed in the control drops (outer buffer drops). The Arf1–Cy3 signal looks lower at the interface between buffer drops because they are adherent and the interface is not vertical, which lowers the integrated intensity. (Scale bar, 50 µm.)