This editorial refers to ‘HSPA12B attenuates cardiac dysfunction and remodelling after myocardial infarction through an eNOS-dependent mechanism’ by J. Li et al., pp. 671–681, this issue.
HSPA12B is a member of a newly identified subfamily of the Hsp70 family of heat shock proteins and is predominately expressed in endothelial cells.1,2 Blood pressure may play a role in its expression, as HSPA12B is expressed in endothelial cells in heart, adipose tissue, brain, kidney, and lung, but not in liver sinusoidal endothelial cells.1 In the heart and brain, vessels of all sizes express HSPA12B, whereas in lung and adipose tissue, expression is largely in capillaries. The cell-restricted expression of HSPA12B, which is otherwise not a feature of heat shock proteins, alone presages its importance to endothelial cell function and indeed studies have established that HSPA12B is required for angiogenesis, specifically in the processes of adhesion, migration, and tube formation.1,2 Endothelial cell HSPA12B expression seems to be dynamic and is increased in confluent HUVEC cultures kept in medium containing endothelial cell-specific growth factors, by heat shock, and during tubule formation.1–3 Protein levels are likely regulated by a post-transcriptional mechanism as well.1 Twenty-two putative client proteins have been identified for HSPA12B, including AKAP12 (A-kinase-anchoring protein 12) and hPODXL (human podocalyxin-like), both of which are implicated in cell adhesion, and aryl hydrocarbon receptor nuclear translocator (ARNT).1
HSPA12B also has protective actions in endothelial cells. Transgenic mice overexpressing the human hspa12b gene (including the endothelial cell-specific promoter) were found to be remarkably protected against endotoxin lipopolysaccharide (LPS)-induced cardiac dysfunction and inflammation.4 In addition, overexpression of human HSPA12B in mice protected against cerebral ischaemia–reperfusion injury.5 In both cases, phosphatidylinositide 3-kinase (PI3K)/AKT signalling, which was either enhanced or preserved and is known to have protective effect in endothelial cells,6,7 was implicated in the beneficial actions of HSPA12B. The mechanism by which HSPA12B impacts on PI3K/AKT signalling in endothelial cells is not known. One possibility may be in supporting the formation of angiopoietin-1 (Ang-1), known to activate PI3K/AKT signalling and have protective actions itself in endothelial cells.4,8 HSPA12B overexpression attenuated the decrease in Ang-1 expression in the heart induced by LPS, although levels were still significantly lower than in saline-treated wild-type and transgenic mice. LPS-induced decrease in eNOS protein levels was also attenuated by HSPA12B overexpression. Altogether, these findings demonstrate that HSPA12B overexpression has protective actions on endothelial cells under stress.
Li et al.3 report that transgenic mice expressing human HSPA12B specifically in endothelial cells exhibited remarkable improvements in cardiac function and remodelling (left ventricular enlargement, wall thinning, and fibrosis) up to 4 weeks after myocardial infarction compared with wild-type mice (Figure 1). Improvements were accompanied by less cardiac myocyte apoptosis and an increase in capillary and arteriolar densities. Transgenic hearts exhibited a further increase in levels of proteins known to have survival actions on cardiac myocytes and endothelial cells and/or to stimulate angiogenesis under conditions of ischaemic stress, namely eNOS, Ang-1, VEGF, and Bcl-2.9–13 Inhibition of eNOS by L-NAME blocked the positive actions of HSPA12B on cardiac function and remodelling, as well as cardiac myocyte apoptosis, VEGF production, and capillary formation. Thus, these events are downstream of, or sustained by, nitric oxide formation. Notably, L-NAME did not prevent the infarct-induced up-regulation of Ang-1, supporting the conclusion that Ang-1 production is a primary event in the protective actions of HSPA12B. Moreover, the observation that HSPA12B overexpression had no effect on eNOS levels in sham animals suggests that HSPA12B impacts on a stress-induced protein rather than an endogenously produced protein.
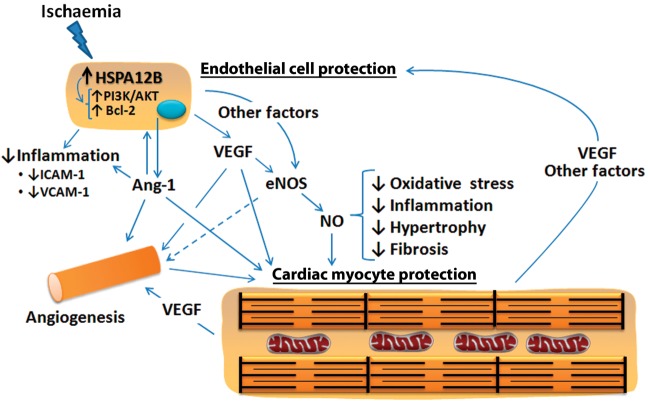
Figure 1.
Pivotal role of endothelial cell-specific heat shock protein HSPA12B in sustaining a protective and reparative response to ischaemic insult in the heart. HSPA12B expression leads to increased generation of Ang-1 and VEGF by endothelial cells that induce new blood vessel formation. Ang-1 has direct anti-inflammatory and protective actions on endothelial cells that involve in part enhanced PI3K/AKT signalling and decreased expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) with reduced immune cell recruitment, and increased expression of anti-apoptotic proteins such as Bcl-2. VEGF is linked to increased eNOS expression and enhanced nitric oxide (NO) formation. NO is known to have actions in the heart that oppose oxidative stress, inflammation, hypertrophy, and fibrosis, thereby reducing adverse cardiac remodelling. Increased NO, VEGF, Ang-1, and angiogenesis would result in the protection of cardiac myocytes and improved cardiac performance. Protected cardiac myocytes, in turn, would secrete VEGF and paracrine factors that protect endothelial cells and favour angiogenesis. HSPA12B likely has direct cytoprotective effects in endothelial cells and contributes to angiogenesis by additional means besides Ang-1 formation. Other not-yet-identified factors are expected to play a role in HSPA12B-related increases in eNOS expression and activity, as well as angiogenesis.
One of the more remarkable features of the study by Li et al. is perhaps its deceptive simplicity, which after all required the identification and exploitation of a cell-specific protein involved in dealing with stress or injury. That is no small feat. On the other hand, simplicity is oftentimes the barometer of utility and is not easily attained. Given the apparent lack of a phenotype of HSPA12B overexpression, it seems likely that HSPA12B, acting as a chaperone protein, enhances a normal reparative process that is ‘turned on’ in endothelial cells by injury. For instance, functioning as a chaperone, HSPA12B may enhance the stress-induced production of Ang-1 by cardiac endothelial cells.
Therapeutic angiogenesis is a promising strategy for tackling chronic myocardial ischaemia and repairing the infarcted heart.14,15 However, delivery of angiogenic factors, in particular VEGF and Ang-1, is fraught with certain difficulties, such as achieving an adequate concentration in the heart, consistency, their short duration of action, and potential adverse or off-site effects such as vascular inflammation and tumour growth.14–16 Targeting angiogenesis using gene therapy addresses some of these concerns, particularly if expression is under control of a promoter that can be turned on and off, such as by hypoxia. However, safety issues remain with gene-based therapies. In addition, angiogenesis is a complex process involving the co-ordinated and temporal actions of a number of angiogenic factors.17 Getting it just right is no easy task. For that reason, exploitation of an endogenous process as achieved in the study by Li et al. is an attractive approach. Whether the formation of mature and functional vessels17 was actually achieved by HSPA12B overexpression will need to be determined.
A better understanding of the factors that regulate HSPA12B expression in the heart may lead to therapeutic strategies to exploit its protective and reparative actions. Li et al.3 did observe that infarction increased HSPA12B expression in wild-type hearts. Like Hsp70, HSPA12B may also have cytoprotective (e.g. anti-apoptotic) actions within endothelial cell through protein–protein interactions.18 That possibility needs to be investigated, as well as whether those intracellular actions are specific to HSPA12B. Overexpressing any protein may introduce spurious interactions.
In conclusion, Li et al. have elegantly demonstrated that enhancing levels of a stress response protein expressed specifically in endothelial cells can concomitantly nurture recovery and attenuate deterioration following ischaemic insult to the heart, thus preserving heart function. Theirs is a ‘simple’ finding that may have profound significance for how ischaemic heart disease is treated.
Conflict of interest: none declared.
Funding
This work was supported by grants from the NIH Institute to G.W.B. (R01HL088101-06) and from the Lebanese University and the National Council for Scientific Research, Lebanon (# 01-10-12) to M.K. F.A.Z. is a recipient of a Research Mini-Fellowship from the Society for Free Radical Biology and Medicine (SFRBM).
References
- 1.Steagall RJ, Rusiñol AE, Truong QA, Han Z. HSPA12B is predominantly expressed in endothelial cells and required for angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:2012–2018. doi: 10.1161/01.ATV.0000235720.61091.c7. [DOI] [PubMed] [Google Scholar]
- 2.Hu G, Tang J, Zhang B, Lin Y, Hanai J, Galloway J, et al. A novel endothelial-specific heat shock protein HspA12B is required in both zebrafish development and endothelial functions in vitro. J Cell Sci. 2006;119:4117–4126. doi: 10.1242/jcs.03179. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zhang Y, Li C, Xie J, Liu Y, Zhu W, et al. HSPA12B attenuates cardiac dysfunction and remodelling after myocardial infarction through an eNOS-dependent mechanism. Cardiovasc Res. 2013;99:671–681. doi: 10.1093/cvr/cvt139. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H, Qian J, Li C, Li J, Zhang X, Ding Z, et al. Attenuation of cardiac dysfunction by HSPA12B in endotoxin-induced sepsis in mice through a PI3K-dependent mechanism. Cardiovasc Res. 2011;89:109–118. doi: 10.1093/cvr/cvq268. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Lu C, Li C, Li R, Zhang Y, Ma H, et al. Overexpression of HSPA12B protects against cerebral ischemia/reperfusion injury via a PI3K/Akt-dependent mechanism. Biochim Biophys Acta. 2013;1832:57–66. doi: 10.1016/j.bbadis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 7.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 8.Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med. 2013;19:31–39. doi: 10.1016/j.molmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Won JY, Lee HY, Lee HJ, Youn SW, Lee JY, et al. Angiopoietin-1 protects heart against ischemia/reperfusion injury through VE-cadherin dephosphorylation and myocardiac integrin-β1/ERK/caspase-9 phosphorylation cascade. Mol Med. 2011;17:1095–1106. doi: 10.2119/molmed.2011.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1α induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036–1044. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu SQ, Tefft BJ, Zhang D, Roberts D, Schuster DJ, Wu A. Cardioprotective mechanisms activated in response to myocardial ischemia. Mol Cell Biomech. 2011;8:319–338. [PubMed] [Google Scholar]
- 12.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu KK. Regulation of endothelial nitric oxide synthase activity and gene expression. Ann NY Acad Sci. 2002;962:122–130. doi: 10.1111/j.1749-6632.2002.tb04062.x. [DOI] [PubMed] [Google Scholar]
- 14.Chu H, Wang Y. Therapeutic angiogenesis: controlled delivery of angiogenic factors. Ther Deliv. 2012;3:693–714. doi: 10.4155/tde.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formiga FR, Tamayo E, Simón-Yarza T, Pelacho B, Prósper F, Blanco-Prieto MJ. Angiogenic therapy for cardiac repair based on protein delivery systems. Heart Fail Rev. 2012;17:449–473. doi: 10.1007/s10741-011-9285-8. [DOI] [PubMed] [Google Scholar]
- 16.Lucerna M, Zernecke A, de Nooijer R, de Jager SC, Bot I, van der Lans C, et al. Vascular endothelial growth factor-A induces plaque expansion in ApoE knock-out mice by promoting de novo leukocyte recruitment. Blood. 2007;109:122–129. doi: 10.1182/blood-2006-07-031773. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Yuan YL, Wang Z, Jiang B, Zhang CS, Wang Q, et al. Sequential, timely and controlled expression of hVEGF165 and Ang-1 effectively improves functional angiogenesis and cardiac function in vivo. Gene Ther. 2013 doi: 10.1038/gt.2013.12. [Published online ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]