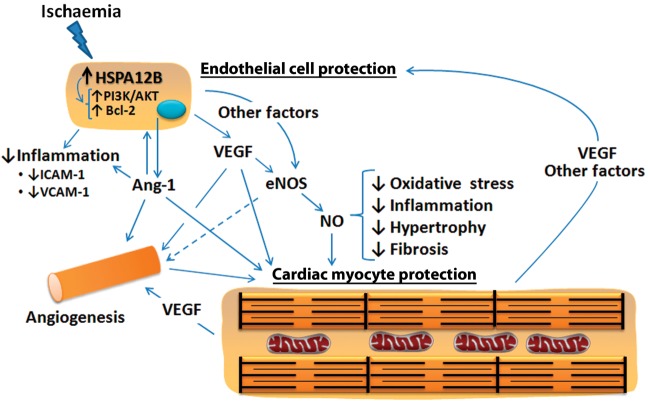
Figure 1.
Pivotal role of endothelial cell-specific heat shock protein HSPA12B in sustaining a protective and reparative response to ischaemic insult in the heart. HSPA12B expression leads to increased generation of Ang-1 and VEGF by endothelial cells that induce new blood vessel formation. Ang-1 has direct anti-inflammatory and protective actions on endothelial cells that involve in part enhanced PI3K/AKT signalling and decreased expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) with reduced immune cell recruitment, and increased expression of anti-apoptotic proteins such as Bcl-2. VEGF is linked to increased eNOS expression and enhanced nitric oxide (NO) formation. NO is known to have actions in the heart that oppose oxidative stress, inflammation, hypertrophy, and fibrosis, thereby reducing adverse cardiac remodelling. Increased NO, VEGF, Ang-1, and angiogenesis would result in the protection of cardiac myocytes and improved cardiac performance. Protected cardiac myocytes, in turn, would secrete VEGF and paracrine factors that protect endothelial cells and favour angiogenesis. HSPA12B likely has direct cytoprotective effects in endothelial cells and contributes to angiogenesis by additional means besides Ang-1 formation. Other not-yet-identified factors are expected to play a role in HSPA12B-related increases in eNOS expression and activity, as well as angiogenesis.