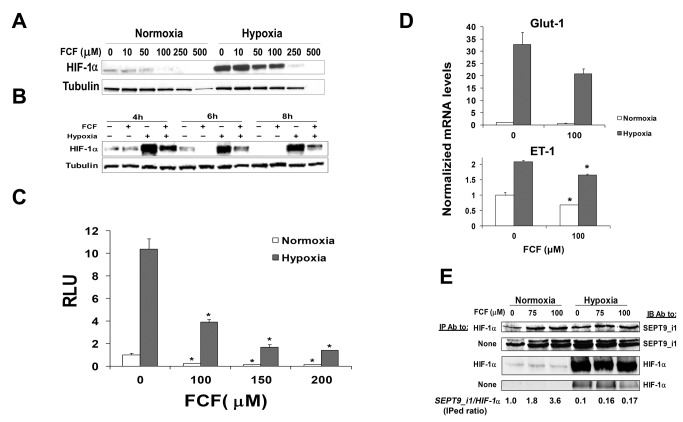
Figure 2. FCF decreases HIF-1α protein expression and HIF-1 transcriptional activity.
PC-3 cells were treated with increasing concentrations of FCF for 6 h (A) or with 100 μM FCF for the indicated times (B) under normoxic and hypoxic conditions. Whole cell extracts were analyzed by SDS-PAGE and immunoblotted with antibodies to HIF-1α and tubulin. (C) PC-3 cells were transiently co-transfected with HRE-dependent firefly luciferase reporter and SV40-dependent renilla luciferase reporter plasmid. After 24 h of transfection, the cells were pretreated with vehicle or 100 µM FCF for 2 h and then grown overnight under normoxia or hypoxia. Whole cell extracts were analyzed by dual luciferase reporter assay. Relative luciferase units (RLU) represent arbitrary units of firefly luciferase activity normalized to renilla luciferase activity. Values were normalized to control vehicle at normoxia. Columns, mean (n = 3); bars, SD; *P < 0.01. (D) PC-3 cells were treated or not treated with 100 μM FCF for 2 h and then subjected overnight to normoxic or hypoxic conditions. Total RNA was isolated from the cells and analyzed by quantitative real-time PCR using primers for Glut-1, ET-1, and cyclophilin B as control. The results were normalized to cyclophilin B mRNA expression levels, and the mean induction of each gene was normalized to control untreated cells under normoxia. Columns, means (n=2); bars, SD; *P < 0.05. (E) PC-3 cells were treated with 0, 75 and 100 μM FCF for 2 h and then subjected to normoxia or hypoxia for an additional 4 h. Cellular extracts were subjected to immunoprecipitation (IP) using anti-HIF-1α antibodies and then immunoblotted (IB) with antibodies to SEPT9_i1 and HIF-1α. None refers to no IP, whole cell extracts only.