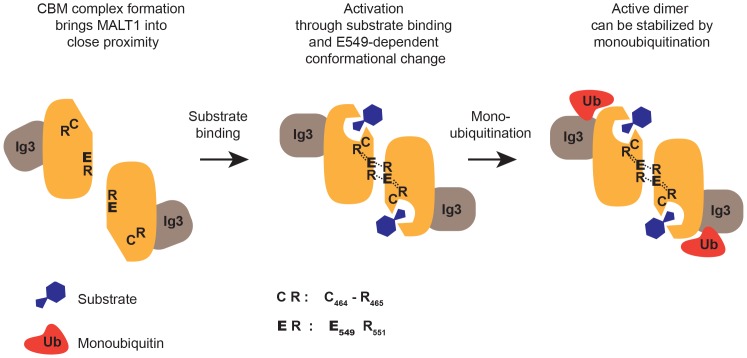
Figure 5. Hypothetical model of MALT1 activation.
Upon antigen receptor activation, MALT1 may dimerize initially by an induced proximity mechanism that could be driven by assembly of the oligomeric CARMA1-BCL10-MALT1 (CBM) complex [43]. Upon substrate binding, E549 (highlighted in bold) in the dimerization interface most likely transmits a conformational change that strengthens dimerization. In this conformation, the active site C464 is reoriented via interaction of R465 with E549, and residues I550 and S552 of opposite subunits interact at the dimerization interface, as suggested by previous crystallographic studies [32], [33]. Our data support the idea that only the dimer in which the two subunits are correctly assembled in this E549-dependent manner can then be stabilized by monoubiquitination. Additional interactions between the α5 and α5′ helices of the two protease subunits that are thought to further stabilize the dimer interaction [33] are not shown in the model.