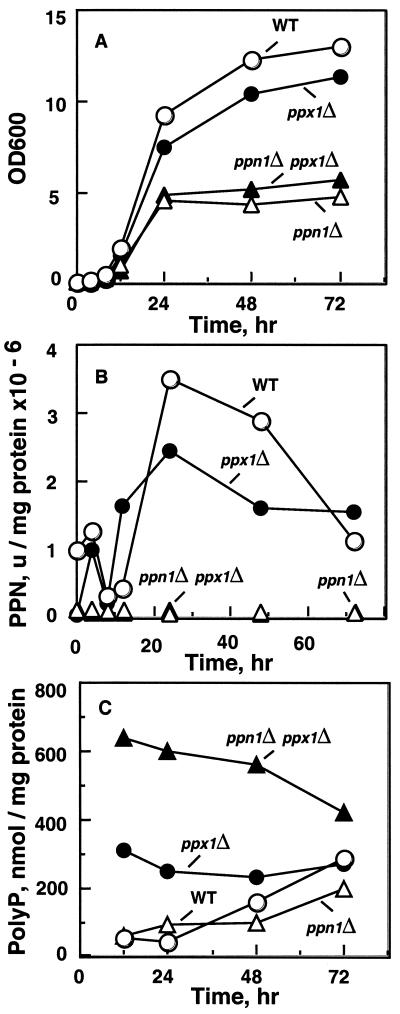
Figure 3.
Yeast strains in YPAD medium. (A) Growth at 30°C. (B) Ppn1-specific activity (106 units/mg of protein) in samples from A. The Ppn1 activity assay was measured as described (15). Crude extracts were prepared from cell pellets suspended in 5 volumes of lysis buffer (0.25 M sucrose/10 mM Tris⋅HCl, pH 7.0/1 mM EDTA) with 250 mg of glass beads. Lysis was obtained by vortexing the suspension 10 times for 30 s each with intermittent cooling on ice. The suspension, after removal of glass beads, was centrifuged at 10,000 × g for 10 min, and the supernatant was used for enzyme assays. A unit of Ppn1 activity was defined as 1 pmol of poly P (as Pi residues) used per minute; units/mg of protein give the specific activity (15). [32P]poly P used as substrate in Ppn1 assays was obtained by synthesis from [γ-32P]ATP using purified E. coli PPK (20); the purified poly P (20) had a chain length of ≈750 Pi residues (P750). (C) Poly P levels in samples from A. Frozen cells were thawed and suspended in equal volume of extraction buffer (50 mM Tris⋅HCl, pH 7.4/100 mM KCl/1 mM EDTA). Cells were lysed as above. Poly P was extracted from the lysate with a buffer of phenol/chloroform saturated with 10 mM Tris⋅HCl, pH 7.5, and 1 mM EDTA, followed by chloroform and ether extractions. Poly P extracted from unlabeled sources was estimated with E. coli PPK (20); poly P extracted from radiolabeled sources was estimated as described (21).