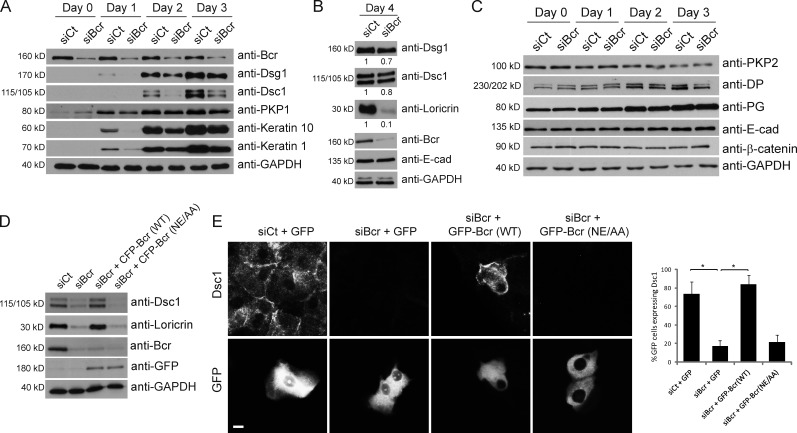
Figure 3.
Loss of Bcr causes a delay in the onset of differentiation markers in submerged cultures of epidermal keratinocytes. (A–C) Control and Bcr KD NHEKs were grown to confluence and switched to high calcium media for 1–4 d to induce differentiation. (A) Control and Bcr KD samples were blotted for Bcr, GAPDH, and a panel of differentiation markers including Dsg1, Dsc1, PKP1, keratin 10, and keratin 1. (B) Samples from lysates taken at 4 d after induction of differentiation were also lysed for Dsg1, Dsc1, loricrin, Bcr, E-cad, and GAPDH. Fold-change values over control quantified by densitometry are noted below blots. These data indicate that Bcr KD causes a decrease in expression of all differentiation markers tested. (C) Samples were also blotted for a range of proteins in the desmosome and adherens junction families, such as PKP2, DP, PG, E-cad, and β-catenin, indicating that expression of cell–cell adhesion proteins is not compromised upon loss of Bcr. All Western blots shown are representative of three independent experiments. (D) Control or Bcr KD NHEKs expressing WT CFP-tagged Bcr or a GEF-dead Bcr mutant (NE/AA) were grown to confluence, induced to differentiate, and samples blotted with Bcr, GFP, Dsc1, loricrin, and GAPDH antibodies (note: anti-Bcr [N-20] antibody does not recognize exogenous expression of CFP-Bcr likely due to interference of the N-terminal CFP tag with the antibody epitope which is at the N terminus of Bcr). (E) Similar samples using GFP-tagged Bcr constructs were induced to differentiate on coverslips and stained for Dsc1. Re-expression of WT Bcr (but not the GEF-dead mutant) can rescue the loss of loricrin and Dsc1 expression seen upon Bcr KD.