FIG 5 .
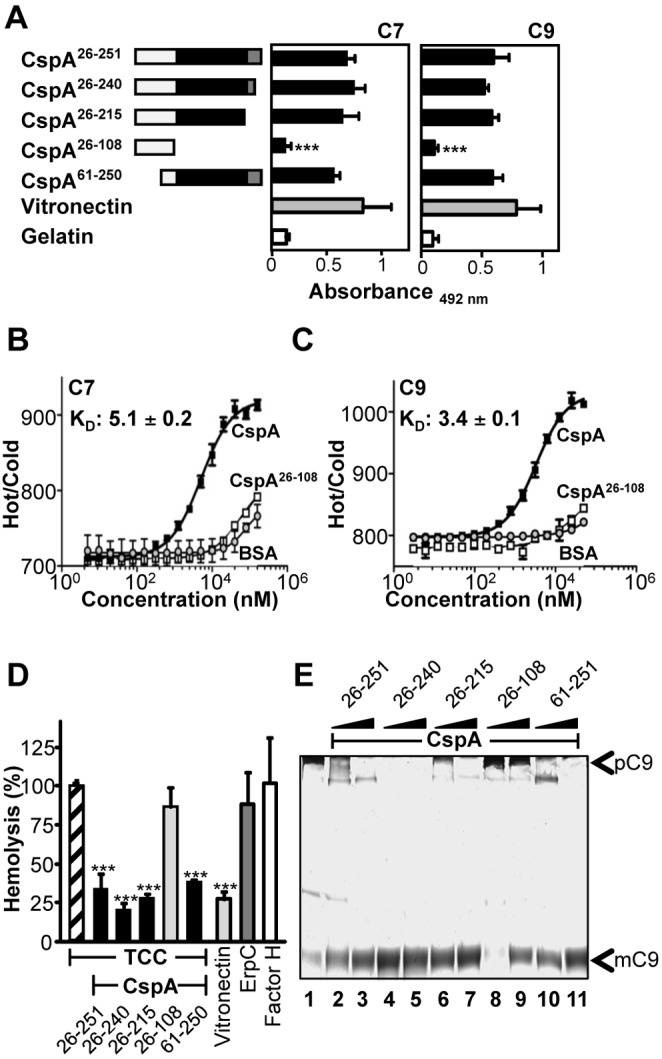
Localization of the TCC inhibitory region in CspA. (A) Full-length CspA26–251 and four deletion mutants were expressed in Escherichia coli and purified. The numbers refer to amino acid residues that are included in each construct (left). Black indicates the C7/C9 binding region, and gray indicates the factor H binding region of CspA (left). Binding of C7 (5 µg/ml) (middle) or C9 (5 µg/ml) (right) to immobilized CspA deletion mutants was assayed by ELISA. Bound C7 was detected with polyclonal C7 antiserum, and bound C9 was detected with polyclonal C9 antiserum followed by HRP-conjugated anti-goat antibody. (B and C) C7 binds to CspA with an affinity of 5.1 ± 0.2 µM and C9 with an affinity of 3.4 ± 0.1 µM. Binding of CspA, CspA26–108, or BSA (0.005 to 160 µM) to NT-647-labeled C7 (12.5 nM) or C9 (12.5 nM) was evaluated in fluid phase by microscale thermophoresis. Thermophoresis was recorded at 80% LED power and 80% MST power for 30 s in a Monilith NT.115 instrument. The relative fluorescence in the thermophoresis phase of the experiment has been plotted against the concentration of CspA. (D) Localization of the region within CspA that mediates TCC inhibition. Full-length CspA26–251 and the four deletion mutants were combined with C7, C8, and C9, and thereafter the mixture was added to C5b-6-coated sheep erythrocytes. Lysis of sheep erythrocytes in the presence of C5b-9 was set to 100%. The mean values from three separate experiments are shown, and error bars show SD. ***, P ≤ 0.001. (E) The amino acid residues 109 to 251 of CspA are relevant for inhibition of C9 polymerization. ZnCl2 induced C9 polymerization, and after incubation the samples were separated by SDS-PAGE; following silver staining, C9 polymers (pC9) and C9 monomers (mC9) were identified by their mobility. C9 polymerizes in the presence of ZnCl2 (lane 1). CspA26–251, CspA26–240, CspA26–215 (lanes 2 to 7), and CspA61–251 (lanes 10 and 11) (2.5 and 5 µg) block polymer formation. CspA26–108 (lanes 8 and 9) does not influence C9 polymerization. The data shown are representative of three independent experiments.
