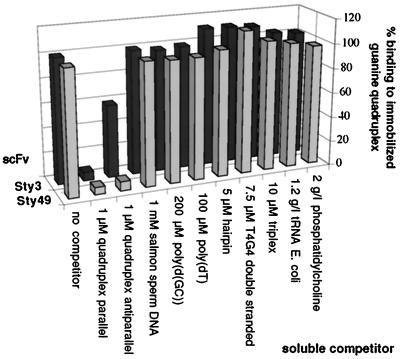
Figure 2.
Analysis of two representative scFv proteins by RIA. mRNA of Sty3 and Sty49 was translated in vitro in the presence of radioactive methionine. After incubation with the listed substrates as competitors, the translation mixture was applied to microtiter plates with immobilized parallel guanine-quadruplex. Each bar represents the average of four measurements. Competitor concentrations are given as follows: per nucleotide for salmon sperm DNA, poly[d(GC)], and poly(dT); per oligonucleotide for hairpin and d(T4G4) double strand; and per base triplet for triplex DNA. The background signal (binding to neutravidin) corresponding to 3–5% of the total signal was subtracted. Nonselected clones did not give a signal above the background. Soluble quadruplex in the parallel conformation abrogated binding of both scFvs Sty3 and Sty49 to the immobilized DNA. Binding was also efficiently inhibited by adding the antiparallel quadruplex conformer to Sty49, but only to a much lower extent in the case of Sty3. None of the other soluble competitors inhibited binding to a detectable degree.