Abstract
OBJECTIVE
Effective interventions to prevent, delay, or remit diabetes are currently available. However, their impact on the prevalence of diabetes at the population level is unknown. This study aimed to estimate the impact of a range of diabetes interventions on the population prevalence of diabetes for Australian adults between 2010 and 2025.
RESEARCH DESIGN AND METHODS
We used the Australian Diabetes Projection Model to estimate the impact of a population-wide strategy, high-risk prevention, surgical diabetes treatment, and a combination strategy on the future population prevalence of diabetes and to estimate the number of diabetes cases that could be potentially prevented in the year 2025.
RESULTS
We estimate that a population-wide strategy would reduce the number of diabetes cases by 60,000–85,000 in 2025 from an estimated 2 million cases under the status quo scenario. A high-risk prevention strategy would result in 106,000 to 150,000 fewer cases of diabetes in 2025, and surgically induced weight loss would result in 3,000–6,000 fewer cases. No single intervention, or combination of interventions, reversed the increasing trend in diabetes prevalence over the next 15 years.
CONCLUSIONS
To reverse upward trends in diabetes prevalence in future years, it is essential that current approaches to diabetes prevention and treatment are optimized and implemented and that alternative approaches to reduce the prevalence of diabetes at a population level are developed.
In Australia, predictions suggest that among adults over 25 years of age, diabetes prevalence will increase from 7.4% (1) in 1999/2000 to 11.4% in the year 2025 (2). This increase is likely to be paralleled by decreasing quality of life, increased morbidity, and increasing healthcare costs.
Effective interventions to prevent, delay, or remit diabetes are currently available. These range from population-wide strategies to alter energy balance or targeted strategies to prevent the progression to diabetes among those at high risk to surgically induced weight loss for those with severe obesity and newly diagnosed diabetes. Population-wide approaches, targeting the whole population regardless of risk, can be achieved through community or regulatory interventions that aim to alter the environment in which unhealthy behaviors occur and incentivize healthy behaviors. However, the evidence base for such policy and intervention is difficult to obtain, and we are currently dependent mostly on modeling studies to draw conclusions regarding effectiveness. On the other hand, landmark randomized controlled trial (RCT) evidence shows that intensive lifestyle interventions can reduce the progression to type 2 diabetes by 58% among those with impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) (3). Real-world translations of these studies have yielded similar results (4,5). Therapeutic interventions such as bariatric surgery have also shown to be effective. RCT evidence demonstrates that weight-loss surgery for obese patients with recently diagnosed type 2 diabetes is associated with a 60–80% remission rate of diabetes (6–8).
The potential impact of implementing these interventions at the population level is not known. Such evidence is essential to inform policy and to ensure that resources are appropriately directed toward diabetes prevention and treatment strategies to reduce the burden of diabetes.
In these analyses, we use a multistate life table model, developed to project the prevalence of diabetes in Australian adults between 2005 and 2025 (2), to estimate the potential impact of a population-wide strategy, a high-risk prevention strategy, and a surgical diabetes treatment strategy on the population prevalence of diabetes for Australian adults between 2010 and 2025.
RESEARCH DESIGN AND METHODS
Overview
We used the Australian Diabetes Projection Model (2) to estimate the impact of four intervention strategies on the future prevalence of diabetes in Australia up until 2025. This model uses data derived from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) and is based on the annual incidence of diabetes as observed between 2000 and 2005 (see Supplementary Appendix and reference 2 for detailed methodological description of the Australian Diabetes Projection Model).
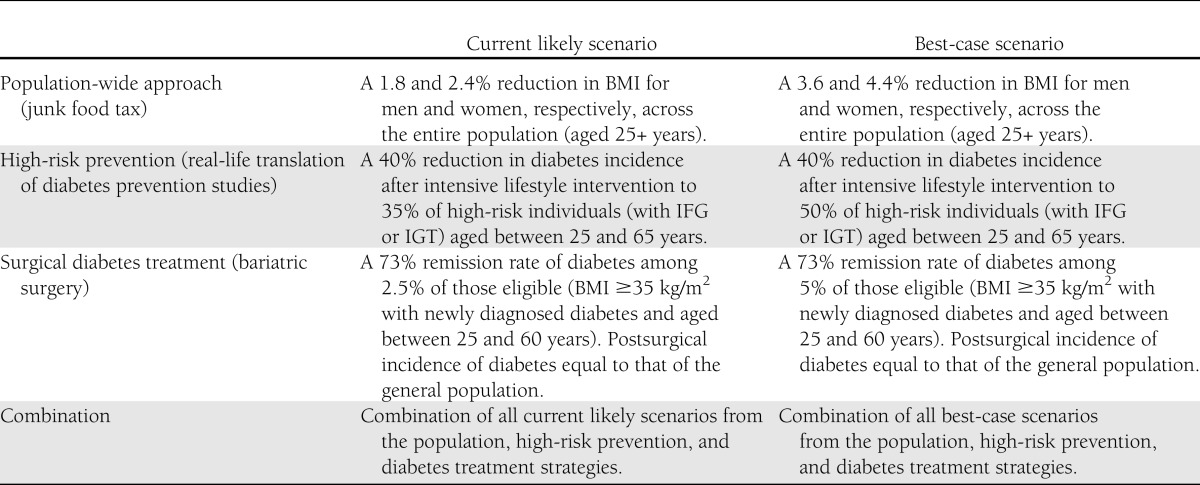
The interventions of interest are described below and summarized in Table 1. Interventions were compared with the status quo scenario whereby the annual incidence of diabetes observed between 2000 and 2005 for the AusDiab cohort remained unchanged for all projected years. All interventions were applied to our model from 2010, and flow-on effects were estimated to 2025. For each of the scenarios 1–4, we estimated the likely impact based on a set of realistic assumptions and a best-case scenario to illustrate the potential impact of each intervention should the uptake or effectiveness of the interventions increase.
Table 1.
Summary of the diabetes prevention and treatment strategies modeled using the Australian Diabetes Projection Model
AusDiab data source
The AusDiab study (9) is a national, population-based survey and examination of 11,247 Australian adults 25 years of age or older at baseline (1999–2000). The response rate to the baseline survey among those who completed a household survey was 55%. In 2004–2005, all participants were invited to a follow-up examination, with a follow-up response rate of 60%. At baseline and follow-up, fasting blood was collected and a 75-g oral glucose tolerance test was undertaken. Diabetes was diagnosed in accordance with the World Health Organization criteria (10). The study was approved by the ethics committee of the International Diabetes Institute.
Modeled scenarios
Population-wide strategy.
To model a population-wide strategy, we used a hypothetical 10% “junk food” tax described in a recent modeling study (11). In this study, the 10% tax was applied to the consumer end price of unhealthy food (where unhealthy food represented noncore foods that are high in saturated fat, sugar, and/or salt and included biscuits, cakes, pastries, pies, snack food, confectionary, and soft drink). The tax was assumed to operate in a similar way to existing Australian excise taxes on alcohol, tobacco, and petroleum in addition to the goods and services tax (11). In our model, the entire population was exposed to this tax and BMI was assumed to reduce by 1.8 and 2.4% for men and women, respectively (derived from the estimated mean reduction in BMI of 0.6 and 0.5 kg/m2 for men and women, respectively) (11) (Table 1).
Linear regression was used to estimate the relationship between fasting plasma glucose and 2-h postload glucose (2hPG) with BMI for men and women and for three age-groups (25–44, 45–64, and 65+ years) in the baseline AusDiab cohort. We used these relationships to reduce 2005 levels of fasting plasma glucose and 2hPG for each participant in the AusDiab cohort accordingly. We then recalculated diabetes (annual) incidence rates for the study population and modeled these as described above. Although we have been specific in the type of population-wide intervention that we have modeled, in reality the junk food tax may represent any population-wide approach that reduces the population level of glucose by a small amount.
In our best-case scenario, we simply doubled the effect size to represent a more optimistic population-wide approach. This resulted in a 3.6 and 4.4% reduction in BMI for men and women, respectively.
High-risk prevention.
To test the population impact of a high-risk diabetes prevention intervention, we reduced the annual incidence of diabetes by an average of 14.0% (apportioned across age categories as observed in the Diabetes Prevention Program [3], resulting in an overall annual reduction of diabetes incidence of 11.2, 14.1, and 17.1% for men and women 25–44, 45–59, and 60–65 years of age, respectively). This estimate was derived from the product of an assumed 35% capture and uptake of high-risk individuals into a prevention program and a mean reduction of diabetes incidence of 40% (Table 1). We based our assumption regarding uptake of intervention on the recent Australian translational studies of the U.S. Diabetes Prevention Program and the Finnish Diabetes Prevention Study (the Melbourne Diabetes Prevention Study, personal communication) (4). In these studies, ∼60% of those approached agreed to be screened, and of those identified at high risk after screening, a further 60% agreed to take part in the intervention (a cumulative capture and uptake of 36%). The assumption of 40% effectiveness was based on randomized controlled trial and real-life translations of high-risk diabetes prevention programs (3,12–15). In this scenario, all individuals aged between 25 and 65 years with IGT or IFG at baseline, or newly diagnosed diabetes at follow-up, were considered eligible for this high-risk prevention intervention, with the assumption that all those with incident diabetes passed through the IGT/IFG state. The intervention effect was assumed to continue as the cohort aged.
In our best-case scenario, we estimated the potential effect of capture and uptake into this program was increased from 35 to 50%.
Surgical diabetes treatment.
Our surgical diabetes treatment strategy was defined as bariatric surgery for anyone aged between 25 and 60 years, with a BMI ≥35 kg/m2 and recently diagnosed diabetes (at or within 2 years of baseline survey), or who developed diabetes over the follow-up period. Of all eligible participants, we assumed that 2.5% in any given year would undergo bariatric surgery, based on current estimated uptakes of this type of surgery in Australia (J. Dixon, personal communication), which was assumed to remain constant for the duration of the model (Table 1). Because there is insufficient evidence describing long-term incidence of diabetes among those who have undergone bariatric surgery, we assumed that individuals who receive bariatric surgery and remitted from diabetes were subsequently exposed to the same diabetes incidence rates as the general population. The benefit of bariatric surgery was derived from a randomized controlled trial demonstrating a 73% remission of type 2 diabetes in obese patients with newly diagnosed diabetes (<2 years) after adjustable gastric banding (6).
In our best-case scenario, we tested the potential effect of increasing the proportion of those who undergo bariatric surgery in any given year to 5% of the eligible population.
Combined intervention approach.
In our combination strategy, we sequentially applied the population-wide, high-risk prevention and surgical diabetes treatment strategies. When applying the surgical diabetes treatment strategy, the proportion of individuals eligible for surgery was assumed not to change from the initial proportion eligible prior to the population-wide and high-risk prevention strategies (Table 1).
RESULTS
Population-wide strategy
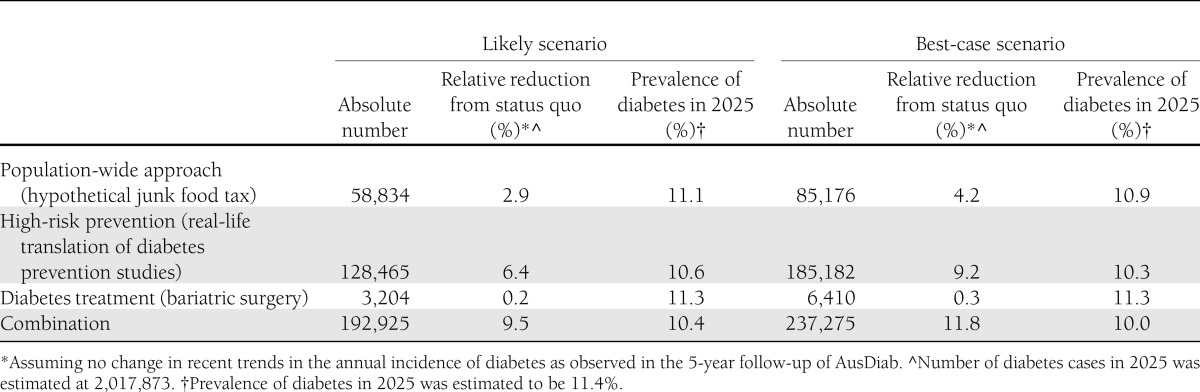
Our modeling of a population-wide intervention demonstrated a reduction in the projected prevalence of diabetes in 2025 from 11.4% under the status quo scenario to 11.1 or 10.9% with likely and best-case scenarios, respectively (Table 2). The likely scenario equated to a reduction of almost 60,000 diabetes cases, or 3% of the 2,017,873 cases that were predicted in 2025. In the best-case scenario, the absolute number of diabetes cases averted was estimated at >85,000 (4.2% relative reduction).
Table 2.
Estimated reduction in the number of diabetes cases in Australia in 2025 after various diabetes prevention and treatment scenarios
High-risk prevention
For the high-risk diabetes prevention intervention, we estimated that in 2025, almost 130,000 cases of diabetes could be prevented, or 6.4% of all diabetes cases compared with the projected status quo scenario. This reduced the 2025 prevalence of diabetes to 10.6% (Table 2). In the best-case scenario, the number of diabetes cases prevented in 2025 was estimated at >185,000, a 9.2% relative reduction. Such a reduction would reduce the prevalence of diabetes in 2025 to 10.3%.
Surgical diabetes treatment
For bariatric surgery (Table 2), we estimated that the population prevalence of diabetes would be reduced to 11.3% in 2025 and that the number of diabetes cases could be reduced by ∼3,000, a relative reduction of 0.2%. Under best-case assumptions, the reduction in the absolute number of diabetes cases in 2025 could be doubled. The population prevalence of diabetes in 2025 would remain relatively unchanged at 11.3%.
Combination approach
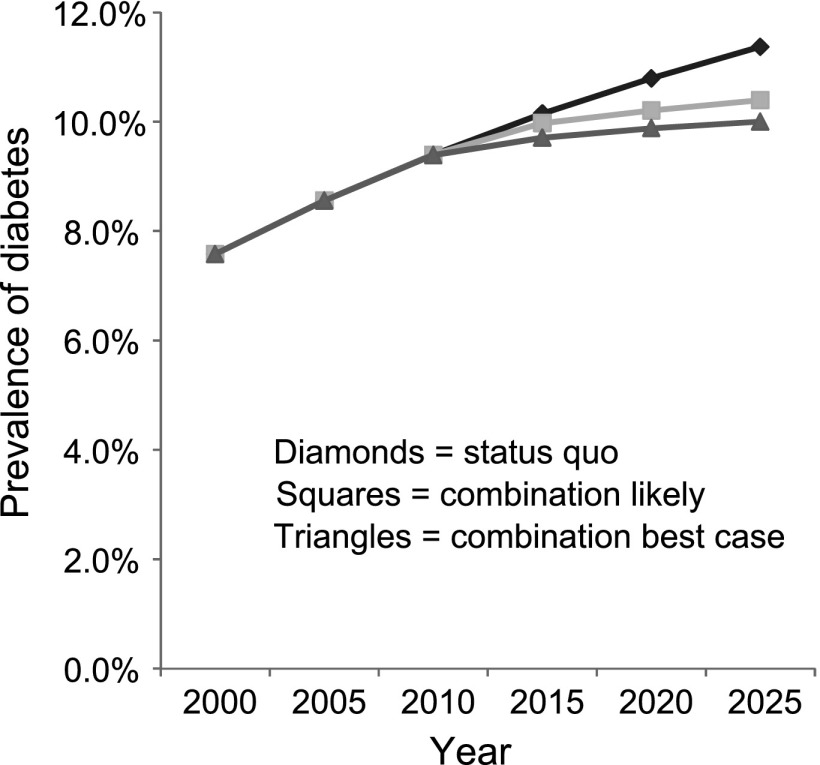
When combining all the current likely scenarios, the population prevalence of diabetes was reduced to 10.4% in 2025, with >190,000 (or 9.5%) fewer diabetes cases (Table 2 and Fig. 1). When combining all the best-case strategies, the prevalence of diabetes could be reduced to 10.0% in 2025. This would reduce the number of diabetes cases by almost 240,000 (representing over one-third of new cases between 2010 and 2025).
Figure 1.
Projected likely and best-case scenario modeling for diabetes prevalence in 2025 under a combination of population-wide, high-risk prevention, and diabetes treatment strategies.
CONCLUSIONS
By modeling the 15-year population impact of a number of diabetes prevention and treatment strategies that are currently being implemented or that are under consideration, we illustrate, for the first time, that the prevalence of diabetes is likely to increase in future years, despite best-practice interventions to prevent or remit diabetes. A combination of interventions, across the spectrum of population-wide strategies, high-risk prevention, and bariatric surgery for severely obese individuals, could result in ∼150,000–200,000 fewer cases of diabetes in 2025. Our modeling of individual diabetes prevention and treatment strategies demonstrates that a high-risk prevention approach would be the most effective single intervention at reducing the future population burden of diabetes, with 106,000 to >150,000 fewer diabetes cases in 2025. The population-wide approach resulted in ∼60,000–85,000 fewer cases of diabetes in 2025. Bariatric surgery reduced the number of diabetes cases by 3,000–6,000. This was not unexpected due to the relatively low number of severely obese individuals with newly diagnosed diabetes.
To our knowledge, only one other study has estimated the effect of diabetes interventions on the future population prevalence of diabetes (16). This study modeled a hypothetical high-risk prevention program among U.S. adults whereby 50% of individuals with IFG were assumed to enter the program and the incidence of diabetes was reduced by 50%. Although this study used slightly more optimistic assumptions than our best-case analysis for high-risk prevention, it nonetheless revealed that such intervention would reduce, but not prevent, future increases in the prevalence of diabetes (16).
The continued increase of diabetes prevalence in the face of best-case diabetes prevention and treatment can be attributed to the relatively high incidence rates combined with the limited reach and efficacy of the interventions modeled. In accordance with the current evidence base, we restricted our high-risk prevention and bariatric surgery approaches to a maximum age of 65 and 60 years, respectively, and it is therefore possible that such approaches miss a large number of incident diabetes cases beyond these ages. However, when testing the effect of implementing these interventions across all ages, the prevalence of diabetes continued to increase (results not shown). Our assumed 35% uptake of high-risk individuals into lifestyle modification programs and the 2.5% of eligible patients who undergo bariatric surgery, derived from existing best-practice evidence, also limit the population impact of these strategies. The limited reach of high-risk prevention strategies may be obviated through improved detection of high-risk individuals and subsequent recruitment and retention in high-risk prevention programs. Similarly, the provision of bariatric surgery could be widened. However, it is important to note that increasing the number of individuals who enter into high-risk prevention programs or who undergo bariatric surgery will require a substantial increase in the capacity of the healthcare system. In regards to effectiveness, with the exception of the population-wide approach, it is unlikely that the interventions modeled will improve dramatically over future years. However, our results indicate the importance of continued improvement in retention and follow-up of those entering high-risk prevention and surgical treatment programs, so that the effect of such interventions are optimized. Furthermore, as the International Diabetes Federation acknowledges, reducing the burden of diabetes will require attention to the creation of an environment that is conducive to healthy food and activity choices (17). Policies are often needed to create such sustainable environments, and although we modeled the effect of one possible policy, it is likely that a suite of regulatory approaches will achieve a larger reduction of population levels of glucose than was assumed in this analysis.
Although a combination of current best-case diabetes interventions only reduced the population prevalence of diabetes by 1.4 percentage points in 2025, the absolute reduction in the number of diabetes cases in 2025 (representing one-third of new cases between 2010 and 2025) is substantial if one considers the costs associated with diabetes. In 2003, the average annual cost per person with type 2 diabetes in Australia was estimated to be between $4,025 and $10,000, depending on the presence and type of associated complications (18).
The strengths of this study include the use of a diabetes projection model that is based on recent estimates of diabetes incidence in the Australian population, the ascertainment of diabetes status in the AusDiab population through the use of oral glucose tolerance tests, the breadth of interventions modeled, and the fact that their effectiveness was drawn from real-world studies wherever possible.
The limitations relate primarily to the underlying assumptions of our model and the quality of evidence related to uptake and persistence of the interventions examined. Our model is conditional on the sustained effect of all interventions for the duration of the model (15 years). It does not take into account the likely increase in diabetes incidence and reductions in mortality that have been observed in other populations worldwide (19,20), nor does it consider any new and effective interventions that may emerge in the future. Furthermore, it is possible that the diabetes incidence rates used in our model are not entirely representative of the Australian population owing to the potential selection bias of the AusDiab cohort, with modest initial and follow-up response rates. However, as we compared all interventions to a status quo scenario that used the same cohort data, this is unlikely to appreciably impact results. In relation to the quality of evidence regarding the assumptions made for our interventions, we were limited by the paucity of empirical evidence describing the effectiveness of a population-wide intervention. We acknowledge that there are many population-wide interventions that we could have modeled that may have led to greater or lesser effects; however, the evidence base for such interventions is lacking. Any population-wide approach to diabetes prevention will typically reduce the level of glucose by a small amount for the entire population, resulting in a left shift in the population distribution of glucose. Indeed, this is what was achieved with our hypothetical modeling of a junk food tax, and thus, in theory, our chosen intervention may in fact represent any population approach. We therefore would not expect our results to be appreciably different regardless of which population approach was chosen to model. Nevertheless, our population-wide intervention modeled herein depended on a recent modeling study that derived the effect size for a junk food tax from the U.K. National Food Survey estimates of price elasticities and the 1995 Australian National Nutrition Survey. As this modeling study predicted a change in BMI associated with a junk food tax, we also relied on the association between BMI and glucose in the baseline AusDiab cohort to obtain a corresponding reduction in glucose levels. Furthermore, our assumption that the subsequent incidence of diabetes after surgical treatment was the same as that observed in the general population needs to be confirmed by empirical data. If the incidence of diabetes is higher in these individuals, it may be expected that we overestimated the benefits of this intervention. However, because of the small number of people who receive surgical treatment in our modeling, the effects of this assumption are likely to be minimal. It is, however, important that models such as the one we present here are consistently updated as new information becomes available.
In addition, the interventions modeled in this study will have additional benefits for health outcomes other than diabetes. For example, epidemiological evidence has demonstrated a continuous positive relationship between blood glucose and cardiovascular disease, well below the diagnostic threshold for diabetes (21). Therefore, even a modest reduction of glucose for a large proportion of the population will likely translate to considerable health benefits. This has particular relevance for our population-wide approach where glucose levels are reduced by a small amount across the entire population. Furthermore, all interventions modeled are likely to translate to improvements in other risk factors, including blood pressure and cholesterol, resulting in additional health benefits that we have not considered.
As the prioritization and implementation of any public health intervention is ultimately determined by its likely costs and benefits, it is essential that future work assesses the cost-effectiveness of each of the interventions evaluated herein. Although the high-risk prevention strategy that we modeled appeared to be most effective under the current assumptions of uptake and effectiveness, given the low costs involved with population-wide interventions, it is likely that the relative cost-effectiveness of interventions may differ.
In conclusion, diabetes prevalence is likely to continue increasing despite public health efforts. It is essential that current approaches to diabetes prevention and treatment are optimized and implemented and that alternative approaches to reduce the population burden of diabetes are developed.
Acknowledgments
This work was supported by a Diabetes Australia Research Trust project grant (Y12P-BACK) and in part by the Victorian Government’s Operational Infrastructure Support Program. D.J.M. is supported by the Victorian Cancer Agency Public Health Fellowship. A.P. received support from a VicHealth Research Fellowship.
No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No potential conflicts of interest relevant to this article were reported.
K.B. designed the analysis and wrote the manuscript. A.P. and D.J.M. designed the analysis, interpreted results, and drafted the article. W.H.H., J.E.S., D.L., and Z.A. interpreted results and drafted the article. K.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are enormously grateful to Anne Allman (Baker IDI Heart and Diabetes Institute), Robert Atkins (Monash University), Stan Bennett (Australian Institute of Health and Welfare), Annaliese Bonney (Baker IDI Heart and Diabetes Institute), Steven Chadban (University of Sydney, Sydney, New South Wales, Australia), Max de Courten (Baker IDI Heart and Diabetes Institute), Marita Dalton (Baker IDI Heart and Diabetes Institute), Terrance Dwyer (Murdoch Children’s Research Institute, Royal Melbourne Hospital), Hassan Jahangir (Baker IDI Heart and Diabetes Institute), Damien Jolley (Monash University), Dan McCarty (Baker IDI Heart and Diabetes Institute), Adam Meehan (Baker IDI Heart and Diabetes Institute), Nicole Meinig (Baker IDI Heart and Diabetes Institute), Shirley Murray (Baker IDI Heart and Diabetes Institute), Kerin O’Dea (University of Melbourne), Kevin Polkinghorne (Monash University), Patrick Phillips (Queen Elizabeth Hospital, Adelaide, South Australia, Australia), Clare Reid (Baker IDI Heart and Diabetes Institute), Alison Stewart (Baker IDI Heart and Diabetes Institute), Robyn Tapp (Baker IDI Heart and Diabetes Institute), Hugh Taylor (Centre for Eye Research, East Melbourne, Victoria, Australia), Theresa Whalen (Baker IDI Heart and Diabetes Institute), and Fay Wilson (Baker IDI Heart and Diabetes Institute) for their invaluable contribution to the setup and field activities of AusDiab.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2501/-/DC1.
References
- 1.Dunstan DW, Zimmet PZ, Welborn TA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002;25:829–834 [DOI] [PubMed] [Google Scholar]
- 2.Magliano DJ, Shaw JE, Shortreed SM, et al. Lifetime risk and projected population prevalence of diabetes. Diabetologia 2008;51:2179–2186 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laatikainen T, Dunbar JA, Chapman A, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health 2007;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Absetz P, Valve R, Oldenburg B, et al. Type 2 diabetes prevention in the “real world”: one-year results of the GOAL Implementation Trial. Diabetes Care 2007;30:2465–2470 [DOI] [PubMed] [Google Scholar]
- 6.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 7.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 8.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012;308:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunstan DW, Zimmet PZ, Welborn TA, et al. Australian Diabetes, Obesity and Lifestyle Study (AusDiab) The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)—methods and response rates. Diabetes Res Clin Pract 2002;57:119–129 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Definition, Diagnosis, and Classification of Diabetes Mellitus and its Complications; Part 1: Diagnosis and Classification of Diabetes Mellitus Geneva, World Health Org., 1999 [Google Scholar]
- 11.Sacks G, Veerman JL, Moodie M, Swinburn B. ‘Traffic-light’ nutrition labelling and ‘junk-food’ tax: a modelled comparison of cost-effectiveness for obesity prevention. Int J Obes (Lond) 2011;35:1001–1009 [DOI] [PubMed] [Google Scholar]
- 12.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care 2011;34:1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating CL, Dixon JB, Moodie ML, et al. Cost-effectiveness of surgically induced weight loss for the management of type 2 diabetes: modeled lifetime analysis. Diabetes Care 2009;32:567–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 15.Dunbar J, Hernan A, Janus E, et al. Melbourne Diabetes Prevention Study research group Implementation salvage experiences from the Melbourne diabetes prevention study. BMC Public Health 2012;12:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Diabetes Federation 2011. IDF Diabetes Atlas, 5 ed. Brussels, Belgium: International Diabetes Federation,
- 18.Colagiuri S, Colagiuri R, Conway B, Grainger D, Davey P. DiabCo$t Australia: Assessing the Burden of Type 2 Diabetes in Australia Canberra, Australia, Diabetes Australia, 2003 [Google Scholar]
- 19.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: a population-based study. Lancet 2007;369:750–756 [DOI] [PubMed] [Google Scholar]
- 20.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB., Sr Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation 2006;113:2914–2918 [DOI] [PubMed] [Google Scholar]
- 21.Brunner EJ, Shipley MJ, Witte DR, Fuller JH, Marmot MG. Relation between blood glucose and coronary mortality over 33 years in the Whitehall Study. Diabetes Care 2006;29:26–31 [DOI] [PubMed] [Google Scholar]