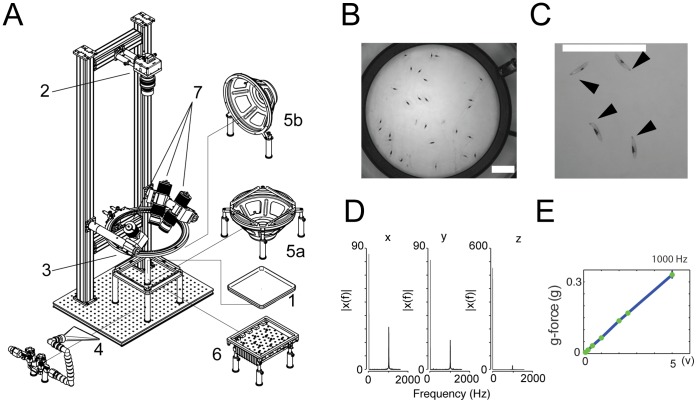
Figure 1. Hardware for somatosensory and optogenetic stimulation of Drosophila larvae.
(A) Larvae roam a plastic dish filled with agar (1). A high-resolution camera (2) collects images to track their movement and body shapes. A ring light (3) provides illumination. Different hardware modules impart stimuli: air current through a 3D-printed flare nozzle (4) connected to plant-supplied compressed air, vibration and sound through a speaker (5a or 5b), blue light for ChR2 activation through an array of high-power blue LEDs (470 nm) underneath the arena (6), and noxious heat through high-power IR light (808 nm) delivered from solid-state lasers (7). (B and C) Snapshots of animals at the start of the experiment on the nociceptive stimulation rig, showing dots for absorption of the 808 nm laser light. Scale bar = 5 cm. Average dot size was 73.28 mm2±4.32 mm2 (s.e.m.). Dots can be placed on the top (B) or on the side (C) to study the directionality of the response. Movie S1 shows larvae with dots on the top rolling in response to 808 nm laser stimulation. Larvae roll in random directions. Movie S2 shows larvae with dots on the left hand side rolling to the left. (D and E) Characterization of the vibration frequency and g-force on agar surface in our rig. (D) Spectral power density plots (|X(f)|) obtained with Fast Fourier Transform (FFT) analysis of the acceleration of the agar surface of the arena when the speaker played 1,000 Hz tones (2 V) (X, Y, Z axis). The spectrum at 1000 Hz is normalized by the spectrum at 0 Hz which represents gravity ( = 1 g). (E) G-force at 1000 Hz increases linearly with increasing voltage applied to the speaker.