Abstract
Numerous roles have been identified for extracellular signals such as Fibroblast Growth Factors (FGFs), Transforming Growth Factor-βs (TGFβs), Wingless-Int proteins (WNTs), and Sonic Hedgehog (SHH) in assigning fates to cells during development of the cerebrum. However, several fundamental questions remain largely unexplored. First, how does the same extracellular signal instruct precursor cells in different locations or at different stages to adopt distinct fates? And second, how does a precursor cell integrate multiple signals to adopt a specific fate? Answers to these questions require knowing the mechanisms that underlie each cell type’s competence to respond to certain extracellular signals. This brief review provides illustrative examples of potential mechanisms that begin to bridge the gap between cell surface and cell fate during cerebrum development.
Introduction
In developmental biology, competence, the ability of cells from one window in time or one part of the embryo to respond to inductive signals, has been appreciated since classical studies involving embryonic tissue transplants [1,2]. The phenomenon of competence also applies to the developing cerebrum, or telencephalon. Transplants of very early telencephalic cells from their normal anterior position in the embryo to more posterior positions reveal transient abilities to change their fates in response to local inductive signals [3,4]. At later stages of telencephalon development, heterochronic and heterotopic transplants of cerebral cortex precursors have also shown that cortical cells from only certain areas or stages are responsive to signals from other cortical areas or stages [5–7]. In addition, genetic approaches are helping to define windows of competence. For example in mice, the ability of telencephalic precursors to adopt a ventral fate in response to SHH was found to be limited to specific stages by conditionally deleting the obligate intracellular effector of SHH signaling, Smoothened, at different times during telencephalon development [8].
Hence it seems that precursor cells change their competence to respond to their environment throughout development of the cerebrum. Although the simple concept of competence has been around since the early days of developmental biology, from what we understand today the potential mechanisms that regulate the ability of a cell to respond to an inductive signal are likely to be complex. These mechanisms will ostensibly include regulated changes in the proteins that carry out or inhibit signal transduction, in combinatorial transcription factor expression, in epigenetic states of chromatin, and in most cases combinations of these.
On the flip side of the responding cells are the inductive signals themselves. These are usually comprised of secreted proteins from a limited number of gene families, including FGFs, WNTs, TGFβs, and in the central nervous system, SHH. These same factors are used repeatedly throughout development to expand, pattern, arrange, differentiate, and wire the nervous system [9–13]. Understanding how the same signals induce such a range of processes remains one of the greater challenges in the field. The answers are likely to involve differences in the combinations and concentrations of these signals and others that are present at any one time or place and, again, differences in the responding cells themselves as enumerated above. At this point in time we have only scratched the surface in terms of understanding the mechanisms for how different cells throughout cerebrum development have distinct responses to the same signal and how cells integrate multiple signals to adopt a fate. Nonetheless, recent studies are providing exciting new insights that are beginning to connect the dots.
Same signal, different effect
Each family of secreted factors is used repeatedly in telencephalon development. A partial list of functions for WNTs and FGFs exemplifies how these factors have different effects on cells depending on their location and developmental stage. WNTs initially inhibit telencephalon formation [14], then maintain cell survival in the early telencephalic primordium [15,16], then play several roles in patterning and cortical neurogenesis (e.g. [17–19]), including acting as an organizer for the hippocampus [20,21], and continue playing roles in regulating neurogenesis in adults [22]. Likewise, numerous roles for FGFs throughout telencephalon development have been uncovered, which include maintaining cell survival during initial telencephalon formation, assigning different positional identities and fates to both ventral and dorsal telencephalic precursors, and regulating several aspects of cortical neurogenesis, wiring, and function [11,12,23].
Although the precise mechanisms explaining how telencephalic precursors respond differently to the same factor depending on their location and developmental stage remain largely unknown, certain types of mechanisms are coming to light. For example, epigenetic changes can partly explain how in response to WNT signaling the competence of cortical precursors to generate neurons rather than astrocytes is reduced or extinguished late in prenatal development. Genes that encode components of the Polycomb group (PcG) complexes, including Ring1B, which function by modifying chromatin structure, were conditionally deleted at early and late stages of cortical development and found to be important for precursor cells to cease generating neuronal cells in response to WNTs and switch to generating astroglial cells [24]. The PcG complex accomplishes this at least in part by inhibiting the expression of a proneurogenic gene, Ngn1, which would otherwise be promoted by WNTs. Given the partial nature of the phenotypes obtained, other mechanisms are also likely to be important for the neurogenic to gliogenic switch. Nevertheless, epigenetic changes will undoubtedly be important in regulating how precursors respond to extracellular signals throughout cortical development and it will be important to identify for each process the pertinent chromatin remodeling factors and functionally relevant sets of genes.
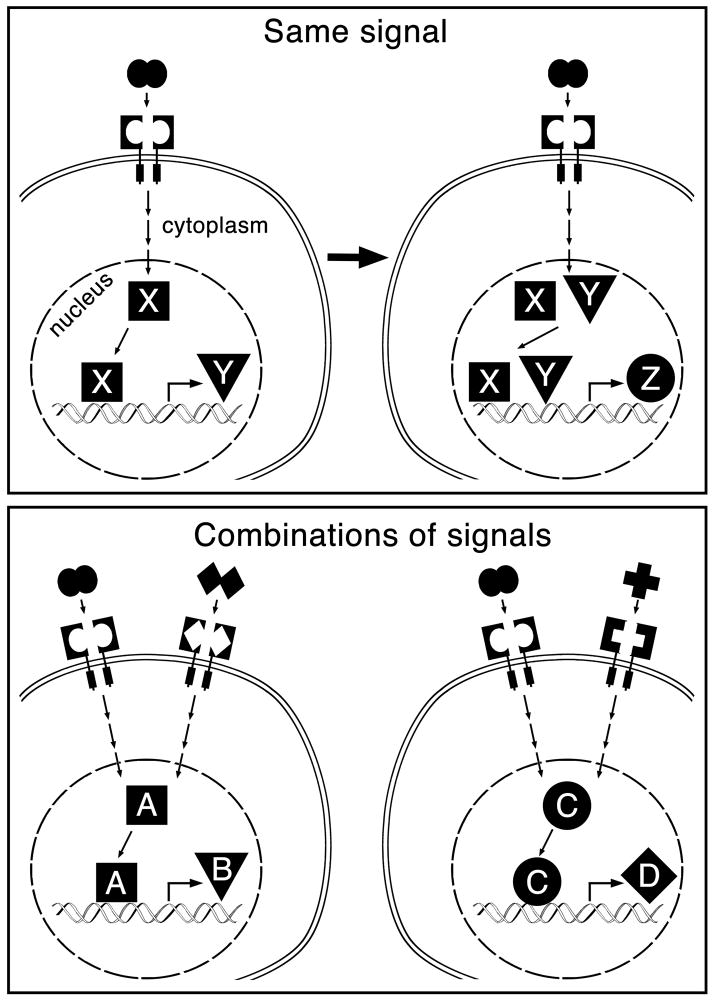
More is known about how cell type-specific transcription factors can influence the responsiveness of telencephalic cells to a particular extracellular signal. Here there are more examples to choose from. For instance, although FGFs, especially FGF8, are expressed in signaling centers at both the anterior end of the neural plate and at the mid-hindbrain boundary [25], they promote Foxg1 expression and telencephalon development in the anterior area [26] while promoting Engrailed expression and midbrain and cerebellar development in the more posterior area [25]. Likewise, SHH is expressed in a signaling center along the entire developing ventral CNS, yet induces different types of ventral neurons in the mid-hindbrain and telencephalic regions [10]. How do FGFs and SHH induce expression of different genes and cell fates in the different areas? The answer comes in part from experiments in chicks showing that the area-specific competence of the cells to respond to these factors is determined by the expression of distinct transcription factors. Namely, Irx3, which is normally expressed in presumptive posterior brain areas of the neural plate, allows precursors to respond to FGF8 and SHH by adopting midbrain fates, while conversely Six3 allows more anterior precursors to adopt telencephalic fates [27]. Development can thus be viewed simplistically as a series of steps in which extrinsic factors progressively restrict the fate of precursor cells by inducing the expression of initial intrinsic factors, which themselves then modify the ability of the cell to respond to the same extrinsic factors, resulting in the expression of secondary intrinsic factors that can then again regulate the response of the cells, and so on (Fig. 1, top).
Figure 1.
Cartoon showing a simplified view of how the same extracellular factor can have different effects on target cells over time (top) and how combinations of factors can also induce different outcomes in target cells (bottom). Letters represent sets of transcription factors, chromatin remodeling factors, and/or signal transduction components, which may or may not be transiently expressed and which affect the response to a signal. In the bottom panel, one signal can modulate another at multiple levels: for example, ligand-receptor interactions, receptor activation, signal transduction, transcription factor interactions, and/or interactions at cis regulatory elements for downstream target genes.
In addition to the expression of specific transcription factors and changes in epigenetic states, a cell’s responsiveness to a signal can potentially be regulated by changes in the expression or function of signal transduction components. Much has been learned from biochemical approaches and cell culture assays about potential intracellular signal transduction pathways for the different families of signals [28–31]. Each family of ligands can potentially activate several intracellular pathways and conversely individual pathways can in some cases be activated by different families of ligands. To complicate things further, crosstalk between pathways can also occur. Hence progress has been slow in identifying the components of intracellular signal transduction that affect the response to a signal in each type of precursor cell in vivo. Nevertheless, examples in tissues other than the developing telencephalon have emerged indicating that the same signal activates different pathways depending on the target cell [12,30]. Such findings underscore the importance of identifying the intracellular transduction pathways that operate in each telencephalic cell type in order to bridge the gap between signals at the cell surface and nuclear factors.
Receiving mixed signals
In addition to the intrinsic differences in the responding cells themselves, a second general explanation for why different precursors have unique responses to the same signal is that other factors in the environment of the cell influence the outcome. In other words, different combinations of extracellular signals have different effects. The question then becomes: how do cells integrate multiple signals to adopt specific fates? Signal integration can occur at many levels, from modulating the activation of receptors at the cell surface to the cross-regulation of signal transduction proteins (Fig. 1, bottom). To date some of the more salient examples in telencephalon development, however, have been integration at the level of cis regulatory elements that drive expression of key fate determinant genes.
One of the earlier examples showed that a combination of WNT and BMP signals drives expression of the transcription factor gene and dorsal telencephalic fate determinant, Emx2 [32]. Emx2 together with Emx1 are essential for the hippocampus to form [33] and Emx2 together with Pax6, another dorsally expressed transcription factor, are essential for forming and patterning the cerebral cortex [34,35]. Binding sites for TCF and SMAD proteins, transcriptional effectors of WNT and BMP signals respectively, were identified within an enhancer that drives expression of Emx2 in the telencephalon [32]. In transgenic assays, these binding sites were necessary and sufficient to promote expression of a reporter, indicating that WNT and BMP signals are integrated at least in part at the level of cis regulatory elements upstream of the Emx2 coding sequence. In this example, WNT and BMP signals are acting cooperatively.
In a more recent example [15], WNT and FGF signals acting antagonistically to TGFβ signals were found to be integrated in part at the cis regulatory elements of Cdkn1a, a gene that promotes cell cycle arrest and apoptotis. At the earliest stages of anterior head development, WNTs directly induce expression of FGF ligands which are required for the survival of telencephalic and face precursors [15,16,36], while TGFβ promote their apoptosis [15]. Two regulatory sites in the upstream region of Cdkn1a, one an enhancer and the other a repressor, integrate these antagonistic signals. When FGF or WNT signals are genetically disrupted, the binding of SMADs, effectors of TGFβ signals, to the enhancer element drives expression of Cdkn1a. When FGFs are present, however, they are sufficient to inhibit Cdkn1a expression due likely to the binding of a MYC-MIZ complex to the repressor element [15,37]. FOXG1, a key determinant of telencephalic fate that is induced by FGFs [26], also participates in regulating Cdkn1a expression by inhibiting the SMAD-binding complex [37].
Moreover, FOXG1 also acts to integrate yet other signals by promoting SHH-mediated ventral telencephalic fates and limiting WNT and BMP-mediated dorsal fates [19,38]. Together with its early role in FGF-mediated telencephalon formation [36,39] and its later role in inhibiting early neuron fates by restricting cortical hem-derived signals [38,40,41], FOXG1 is not only a favorite telencephalic marker, but also a good example of how one molecule can play central roles in coordinating multiple steps during development (Fig. 2).
Figure 2.
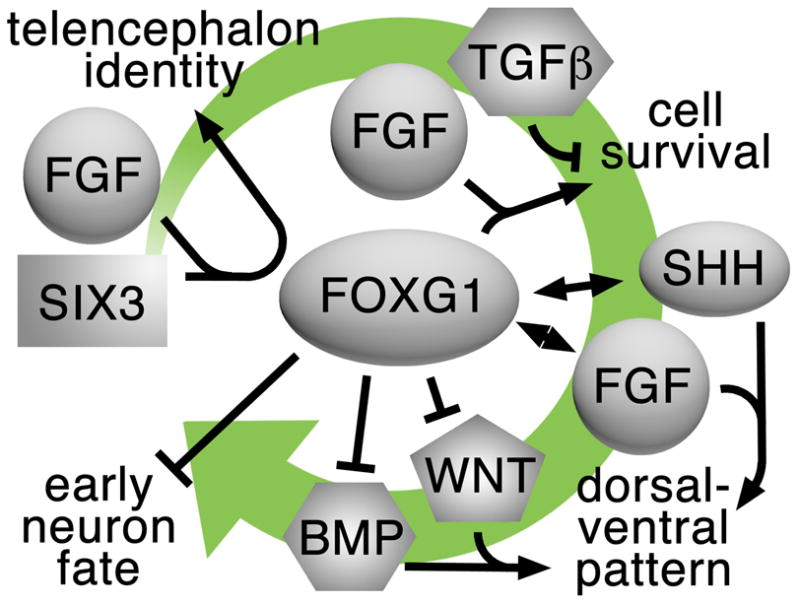
FOXG1 is presented as an example of a central factor that integrates or coordinates multiple signals during several steps of telencephalon development. These steps include telencephalon specification, cell survival, dorsal-ventral patterning, and the regulation of neurogenesis. The green arrow represents the direction of development from early to late.
Small molecule, big impact?
It is clear that deficiencies in the extracellular signals discussed here can lead to serious developmental brain defects, but even more minor imbalances that alter the numbers, positions, ratios, or maturation of different neural cell types have been proposed to underlie psychiatric disorders [42–45]. At some point, intervening in abnormal developmental processes of the cerebrum for therapeutic purposes might be considered. Small molecules, for example, could be used to activate or inhibit specific signaling pathways. However, because of the variety of ways in which different cells respond to the activation of the same pathways, whether due to their environment or their inherent competence, the effects of using small molecules to regulate precursors in vivo might be difficult to predict. Nevertheless, the topics of inductive signals and competence, signal transduction in vivo, and signal integration are ripe for study and will greatly further our understanding of fundamental processes in forebrain development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Waddington CH. Organisers and Genes. Cambridge: Cambridge University Press; 1940. [Google Scholar]
- 2.Wolpert L. Principles of Development. Oxford: Oxford University Press; 1998. [Google Scholar]
- 3.Cox WG, Hemmati-Brivanlou A. Caudalization of neural fate by tissue recombination and bFGF. Development. 1995;121:4349–4358. doi: 10.1242/dev.121.12.4349. [DOI] [PubMed] [Google Scholar]
- 4*.Baizabal JM, Valencia C, Guerrero-Flores G, Covarrubias L. Telencephalic neural precursor cells show transient competence to interpret the dopaminergic niche of the embryonic midbrain. Dev Biol. 2011;349:192–203. doi: 10.1016/j.ydbio.2010.11.003. A study showing that classic transplant experiments are still informative. [DOI] [PubMed] [Google Scholar]
- 5.McConnell SK. The control of neuronal identity in the developing cereberal cortex. Curr Opin Neurobiol. 1992;2:23–27. doi: 10.1016/0959-4388(92)90156-f. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary DDM, Schlaggar BL, Tuttle R. Specification of neocortical areas and thalamocortical connections. Annu Rev Neurosci. 1994;17:419–439. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- 7.Levitt P, Barbe MF, Eagleson KL. Patterning and specification of the cerebral cortex. Annu Rev Neurosci. 1997;20:1–24. doi: 10.1146/annurev.neuro.20.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Sousa VH, Fishell G. Sonic hedgehog functions through dynamic changes in temporal competence in the develoiping forebrain. Curr Opin Genet Dev. 2010;20:391–399. doi: 10.1016/j.gde.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirabayashi Y, Gotoh Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci Res. 2005;51:331–336. doi: 10.1016/j.neures.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 11.Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Hébert JM. FGFs: neurodevelopment’s Jack-of-all-trades, how do they do it? Front Neurosci. 2011;5:133. doi: 10.3389/fnins.2011.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiberi L, Vanderhaeghen P, van den Ameele J. Cortical neurogenesis and morphogenesis: diversity of cues, sources and functions. Curr Opin Cell Biol. 2012;24:269–276. doi: 10.1016/j.ceb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Paek H, Hwang JY, Zukin RS, Hébert JM. β-Catenin-dependent FGF signaling sustains cell survival in the anterior embryonic head by countering Smad4. Dev Cell. 2011;20:689–699. doi: 10.1016/j.devcel.2011.04.010. This study explains how a balance between different signaling pathways is integrated to regulate cell survival and the expression of a key gene in the early telencephalon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Song L, Zhou CJ. The canonical Wnt/β-catenin signaling pathway regulates Fgf signaling for early facial development. Dev Biol. 2011;349:250–260. doi: 10.1016/j.ydbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 18.Zhou CJ, Borello U, Rubenstein JL, Pleasure SJ. Neuronal production and precursor proliferation defects in the neocortex of mice with loss of function in the canonical Wnt signaling pathway. Neurosci. 2006;142:1119–1131. doi: 10.1016/j.neuroscience.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 19*.Danesin C, Peres JN, Johansson M, Snowden V, Cording A, Papalopulu N, Houart C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev Cell. 2009;16:576–587. doi: 10.1016/j.devcel.2009.03.007. This study underscores the importance of Foxg1 in coordinating and integrating multiple signals during telencephalon development. [DOI] [PubMed] [Google Scholar]
- 20.Lee SMK, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 21.Mangale VS, Hirokawa KE, Satyaki PRV, Gokulchandran N, Chikbire A, Subramanian L, Shetty AS, Martynoga B, Paul J, Mai MV, Li Y, Flanagan LA, Tole S, Monuki ES. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and Retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwata T, Hevner RF. Fibroblast growth factor signaling in development of the cerebral cortex. Dev Growth Differ. 2009;51:299–323. doi: 10.1111/j.1440-169X.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 24**.Hirabayashi Y, Suzki N, Tsuboi M, Endo TA, Toyoda T, Shinga J, Koseki H, Vidal M, Gotoh Y. Polycomb limits the neurogenic competence of neural precursor cells to promte astrogenic fate transition. Neuron. 2009;63:600–613. doi: 10.1016/j.neuron.2009.08.021. This paper provides a rare example of how a chromatin remodeling factor regulates the competence of neocortical precursors to respond to WNTs and transition from neurogenic to gliogenic. [DOI] [PubMed] [Google Scholar]
- 25.Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 26.Hébert JM, Fishell G. The genetics of telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129:89–93. doi: 10.1242/dev.129.1.83. This paper reveals how two transcription factors underlie the competence of neural precursor cells to respond differently to the same signals. [DOI] [PubMed] [Google Scholar]
- 28.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytok Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. This review provides a compeling perspective on the complexities of how cells interpret a TGFβ signal depending on their context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusse R. Wnt signaling. Cold Spring Harb Perspect Biol. 2012;4(5) doi: 10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. This early study provides what is still one of the few examples of how multiple signals directly converge on the cis regulatory elements of a telencephalic fate-determinant gene. [DOI] [PubMed] [Google Scholar]
- 33.Shinozaki K, Yoshida M, Nakamura M, Aizawa S, Suda Y. Emx1 and Emx2 cooperate in initial phase of archipallium development. Mech Dev. 2004;121:475–489. doi: 10.1016/j.mod.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Bishop KM, Goudreau G, O’Leary DDM. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 35.Muzio L, DiBenedetto B, Anastassia S, Boncinelli E, Gruss P, Mallamaci A. Conversion of cerebral cortex into basal ganglia in Emx2−/− Pax6Sey/Sey double-mutant mice. Nat Neurosci. 2002;5:737–745. doi: 10.1038/nn892. [DOI] [PubMed] [Google Scholar]
- 36.Paek H, Gutin G, Hébert JM. FGF signaling is strictly required to maintain early telencephalic precursor cell survival. Development. 2009;136:2457–2465. doi: 10.1242/dev.032656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Seoane J, Le HV, Shen L, Anderson SA, Massagué J. Integration of Smad and Forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. With a focus on the regulation of a single gene, the authors of this paper were able to provide a mechanism for how the integration of multiple signaling pathways regulate its expression in neural cells. [DOI] [PubMed] [Google Scholar]
- 38.Dou CL, Li S, Lai E. Dual role of Brain Factor-1 in regulating growth and patterning of the cerebral hemispheres. Cer Cortex. 1999;9:543–550. doi: 10.1093/cercor/9.6.543. [DOI] [PubMed] [Google Scholar]
- 39.Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 40.Hanashima C, Fernandes M, Hébert JM, Fishell G. The role of Foxg1 and dorsal midline signaling in the generation of Cajal-Retzius subtypes. J Neurosci. 2004;27:11103–11111. doi: 10.1523/JNEUROSCI.1066-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2007;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- 42.Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Vaccarino FM, Grigorenko EL, Smith KM, Stevens HE. Regulation of cerebral cortical size and neuron number by fibroblast growth factors: implications for autism. J Autism Dev Disord. 2009;39:511–520. doi: 10.1007/s10803-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]