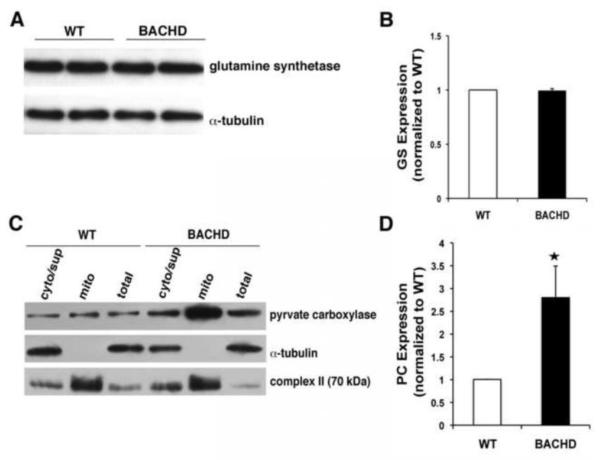
Figure 4. Pyruvate carboxylase expression is increased while glutamine synthetase is unaltered in BACHD astrocytes.
(A) Representative Western blot probing glutamine synthetase levels in astrocytes. Immunoreactivity of α-tubulin was used as a control for gel loading. (B) Quantification of glutamine synthetase (GS) levels (n=4) shows no difference between BACHD and WT astrocytes. (C) Representative Western blots of total, cytosolic (i.e. non-mitochondrial supernatant; cyt/sup) and mitochondrial (mito) protein in BACHD and WT astrocytes probing pyruvate carboxylase. Immunoreactivity of cytosolic α-tubulin and mitochondrial complex II were used to confirm success of our fractionation procedure. Note that the aggressive mitochondrial purification (exclusion) approach results in some contamination of the cytosolic/supernatant fraction by mitochondrial proteins. (D) Quantification of pyruvate carboxylase (PC) levels show ~3-fold increase in BACHD mitochondria (n=4 mice, Mann-Whitney U-test, *p <0.03). Data shown as mean ± SEMs.