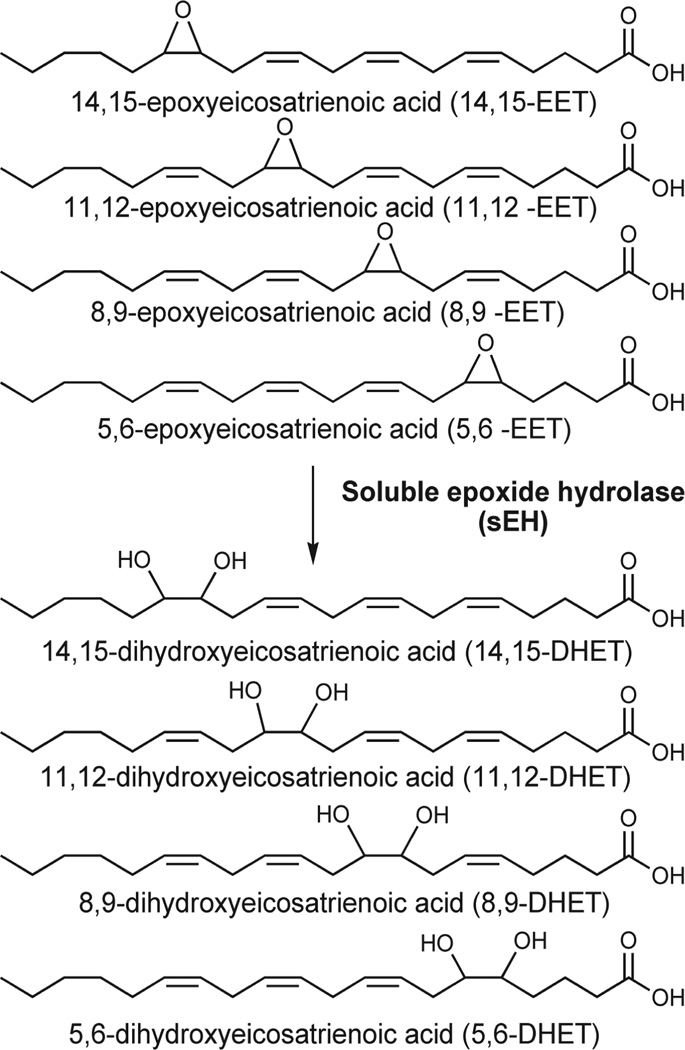
Figure 1.
Structures of epoxyeicosatrienoic acids (EETs) and dihydroxyeicosatrienoic acids (DHETs). The largely antihypertensive, anti-inflammatory, and analgesic EETs are converted to their corresponding diols through the action of soluble epoxide hydrolase (sEH). The diols are more easily conjugated, more water-soluble, and easier to excrete. The diols also have greatly reduced biologic activity. Thus the overall effect of sEH hydrolysis of these compounds is to decrease the epoxylipid signal.