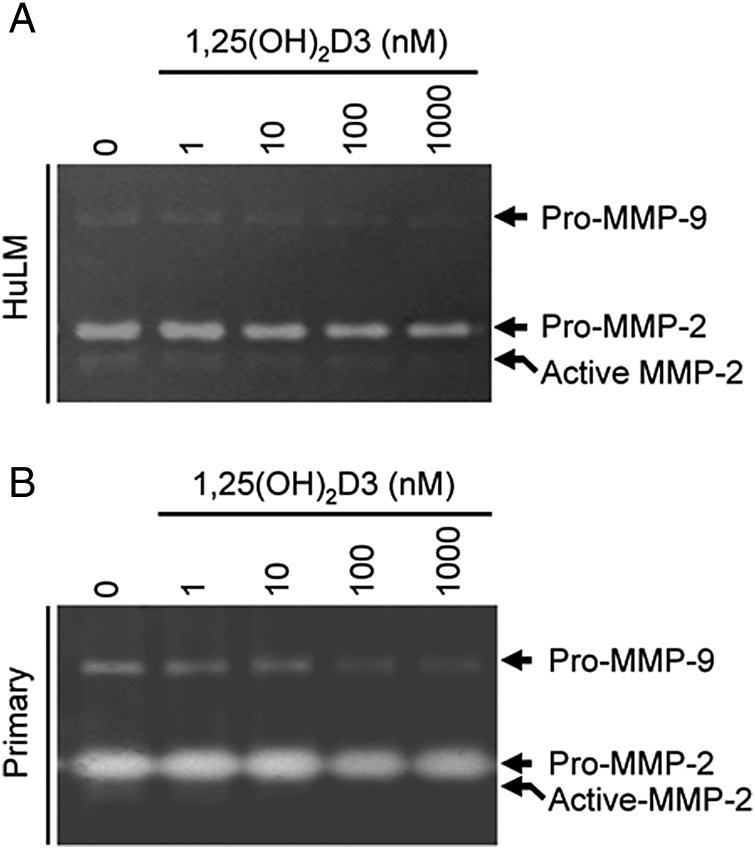
Figure 6.
Effect of 1,25(OH)2D3 on gelatinolytic activities of MMP 2 and 9 in cultured human uterine fibroid cells. Both immortalized human uterine fibroid (HuLM) cells (A) and primary uterine fibroid cells (B) were serum starved and treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 M and 1000 nM) for 48 h. Each supernatant medium (40 µl/each sample) was analyzed in 7.5% SDS PAGE gelatin zymography as described in the section Materials and methods. The digestion of gelatin in the gel by MMP-2 and MMP-9 enzymes cause the negative (unstained) bands. The pro-MMP-2 (72 kDa) and active MMP-2 (65 kDa) digested bands were detected in the gels which were decreased by 1,25(OH)2D3. Similarly, the pro-MMP-9 (92 kDa) was also detected in supernatant media in both cell types, which were also decreased by 1,25(OH)2D3. The active form of MMP-9 was undetectable in supernatant media of both cell types. This experiment was repeated twice with similar results.