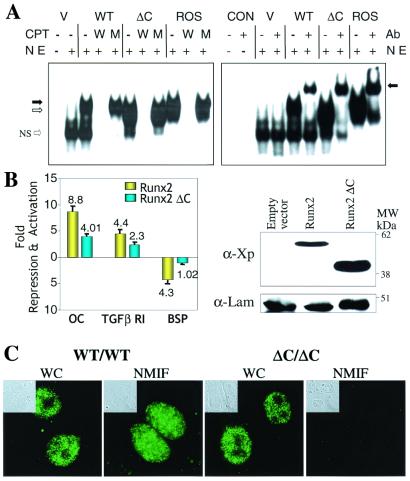
Figure 5.
Functional properties of the Runx2ΔC protein. (A) Runx2ΔC protein retains DNA binding activity. (Left) HeLa cell nuclear extracts (NE) were prepared 24 h after transfection with empty vector (V), WT, or Runx2ΔC mutant protein expression plasmids. Nuclear extracts from Ros 17/2.8 cells that endogenously express Runx2 provided a positive control. An oligonucleotide containing the Runx consensus sequence was used as labeled probe in all lanes. Competitor lanes (CPT) contained 100-fold excess of either WT (W) or mutant (M) Runx binding site oligonucleotides compared with control without specific competitor (−) are indicated. The arrows indicate the specific protein-DNA complexes for full-length (solid arrow) and truncated (open arrow) Runx2; NS represent a nonspecific band. (Right) Gel mobility-immunoshift analysis with (+) or without (−) the addition of 1 μl polyclonal Runx2 antibody (Ab) to detect WT and Runx2ΔC (ΔC) mutant proteins transiently expressed in HeLa cells. CON designates two control lanes for probe alone and probe plus antibody. V represents HeLa cell nuclear extracts transfected with the empty vector. The arrow indicates the supershifted complex. (B) Functional activity of the WT and ΔC Runx proteins on Runx-dependent promoters. (Left) HeLa cells were cotransfected with −1,097/+23 rat osteocalcin (OC), −1.0-kb transforming growth factor β R1, or −620/+25 bone sialoprotein (BSP) promoter-chloramphenicol acetyltransferase constructs together with either X-press-tagged WT Runx2 or Runx2ΔC expression plasmids (40–42). Promoter activities were determined 24 h posttransfection. Studies were repeated twice with n = 6 replicates with two different DNA preparations. Data were normalized to luciferase values. (Right) Western blot analysis of lysates from transfected cells from the same experiment. The X-press-tagged WT and mutant Runx proteins were detected by using α-X-press antibody from Invitrogen. Lamin B shows equal protein loading. (C) In vivo targeting of Runx2ΔC to subnuclear sites is compromised. Cells were obtained from calvarial tissue of WT and homozygous ΔC mutant mice at 17.5 dpc cultured for 2 days on glass coverslips and prepared for in situ immunohistochemistry of WT and Runx2ΔC proteins. WT cells (×100) show punctate staining of Runx2 in whole cells (WC), which is retained in the nuclear scaffold/NMIF preparations. Homozygous (ΔC/ΔC) cells (×100) also show distinct punctate foci in whole cells, but complete absence of Runx2ΔC in the NMIF preparations. The antibody control (no primary antibody) shows background fluorescence for comparison (Fig. 7, which is published as supplemental material for this data). Phase microscopy (Insets) shows cellular and nuclear morphology in WC and NMIF. Nuclei were stained with 4′,6-diamidino-2-phenylindole (0.5 μg/ml) to verify removal of DNA during the NMIF extraction procedure (see Fig. 7 for this data).