Abstract
Objectives:
This study aimed to investigate the association between pre-pregnancy maternal body mass index (BMI), gestational weight gain and low birth weight (LBW) in babies born to a sample population of Omani women.
Methods:
A case-control study was carried out among deliveries registered between 1st May 2010 and 30th April 2011 at Sultan Qaboos University Hospital, Muscat, Oman. A case was defined as a woman who delivered a low birth weight baby (<2,500 g); a control was a woman delivering a baby weighing between 2,500 and 4,000 g. A random selection of 150 cases and 300 controls was carried out using the hospital information system. Maternal, pre-natal, and delivery data were extracted from the mothers’ follow-up cards. Bivariate and multivariate logistic regression analyses were executed to examine the association between pre-pregnancy maternal BMI and LBW.
Results:
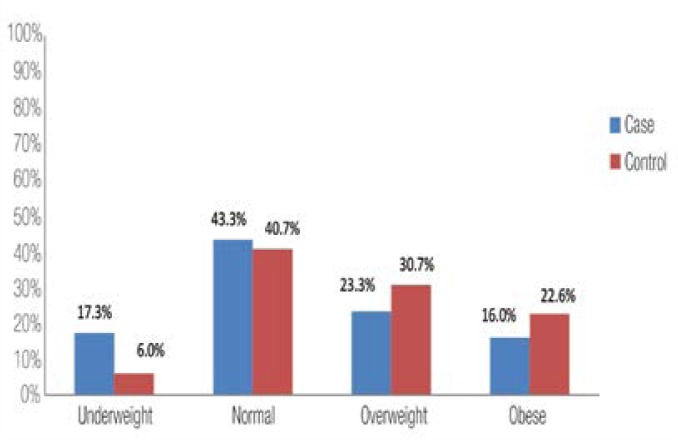
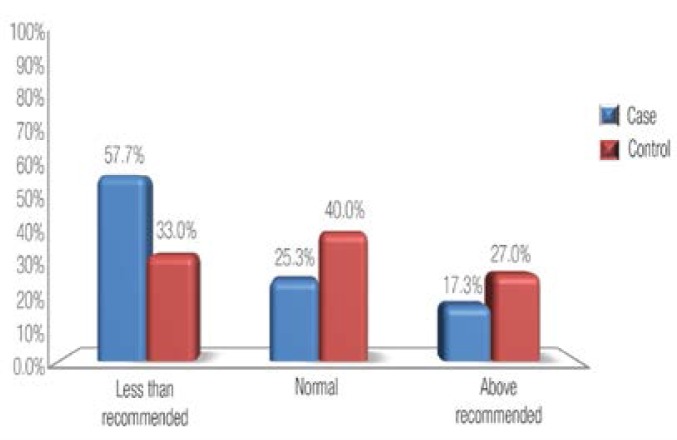
The percentage of underweight mothers (BMI <18.5) was higher among the cases compared to the controls (17.3% versus 6%; P <0.001). The proportion of mothers with less-than-recommended weight gain was also higher among the cases compared to the controls (57.7% versus 33%; P <0.001). After adjustment for potential confounders, infants of underweight mothers had more than twice the risk of LBW compared to those of mothers with normal weight (odds ratio = 2.27; 95% confidence interval 1.09–4.71).
Conclusion:
Underweight Omani women as well as women with less-than-recommended gestational weight gain were at higher risk of delivering LBW babies. Maternal health promotion programmes should be directed towards improving mothers’ nutrition before and during pregnancies.
Keywords: Body Mass Index, Pregnancy, Gestational Age, Birth Weight, Oman
Advances in Knowledge
- A mother’s weight before and during pregnancy influences her baby’s birth weight.
Application to Patient Care
- Prenatal and antenatal nutritional counselling is essential to minimise the risk of women delivering low birth weight babies.
The World Health Organization (WHO) defines low birth weight (LBW) as weight at birth of less than 2,500 g. Infants with LBW are approximately 20 times more likely to die than heavier babies.1 LBW affects a person throughout life and is associated with poor growth in childhood and, subsequently, a higher incidence of adult diseases, such as type 2 diabetes (DM), hypertension (HTN) and cardiovascular disease.2
At the population level, LBW is an indicator of numerous public health problems, such as long-term maternal malnutrition, ill health, overwork and poor care during pregnancy. At the individual level, LBW is a predictor of newborn health and survival. Furthermore, in healthcare scenarios, the success of a maternal-child health programme can be evaluated and monitored using the incidence of LBW babies as a key indicator.3
In developing countries, 19 million babies (16%) per year are born with LBW. Asia has the highest incidence of low birthweight, with 18% of all babies weighing under 2,500 grams at birth. Mauritania, Pakistan, the Sudan and Yemen all have an estimated low birthweight incidence of more than 30%. However, the figures may be much higher since 60% newborns in developing countries are not weighed at birth.2 LBW can result from prematurity, fetal growth restriction or fetal pathology, or it can be constitutional.
The nutritional status of women plays a crucial role for the well-being of both the mother and their developing fetuses. Two independent factors—pre-maternal body mass index (BMI) and weight gain during pregnancy—play important roles in determining the outcome of the pregnancy for both the mother and fetus.4 Various reports have documented the significant effect of these two factors and their impact on the outcomes of pregnancy, especially in developing countries.5–7
Oman is a developing nation on the Arabian Peninsula, with a population of around 3 million, but the Omanis constitute only 65% of the population according to recent statistics.8 In Oman, the incidence of LBW was estimated to be around 9.2% in 2009 compared to 8.1% in 2000.9 Omani children with LBW are at a higher risk of being underweight when they grow up.10 This study aimed to evaluate the association of maternal BMI before pregnancy and gestational weight gain on LBW babies in a cohort of Omani women at Sultan Qaboos University Hospital (SQUH), a tertiary teaching hospital in Muscat, Oman.
Methods
A case-control study was carried out among Omani women who delivered at SQUH from the 1st May 2010 to 30th April 2011. Both the cases and the controls were identified using the computerised hospital information system. A list of deliveries at SQUH during the study period was generated and organised into two groups: cases and controls. A case was defined as a woman who delivered a LBW baby (<2,500 g) and a control as a woman delivering a baby weighing 2,500–4,000 g. A total of 150 cases and 300 controls were selected based on an expected prevalence of maternal underweight among LBW babies (15%) being two times higher than the prevalence of maternal underweight among normal weight babies, with a power of 80% and an error (a) of 5%.10 The allocation ratio was 1:2. Subjects were selected using systematic sampling of every fifth case and of every ninth control.
Medical record numbers (MRN) of cases and controls were used to extract maternal, prenatal and delivery data from the mothers’ follow-up cards. Variables included in the data collection were maternal age, pre-pregnancy BMI, gravidity, gestational age and weight gain, past medical history, antenatal complications, the baby’s gender and mode of delivery.
The pre-pregnancy weights of mothers were obtained from the mothers’ health cards if their first prenatal visit was before 13 weeks’ gestation, as weight does not change in the first trimester. Pregnant women with a first visit made after 13 weeks’ gestation were excluded to avoid errors in the measurement of weight and height. Then, pre-pregnancy BMI was calculated as pre-pregnancy weight in Kg divided by measured height in metres squared.11 The WHO definitions of BMI for the Omani population were used as follows: underweight (BMI <18.5), normal (BMI ≥18.5 and <25), overweight (BMI ≥25 and <30), and obese (BMI ≥30).11 The classifications of the US Institute of Medicine (IOM) for recommended gestational weight gain were used according to specific BMI.12
Means and standard deviations for parametric variables and frequencies and percentages for categorical data were described. The two sample proportions were tested with the chi-square test. Both bivariate and multivariate logistic regression analyses were carried out using Statistical Package for the Social Sciences (SPSS), Version 19 (IBM Corp., Chicago, Illinois, USA) to examine the association between LBW, maternal BMI and gestational weight gain with adjustment of potential confounders such as pregnancy-induced HTN or prematurity. All high-risk pregnancies, for example those complicated by preeclampsia, systemic lupus erythematosus (SLE) and HTN were followed-up with fetal Doppler scans. The study was approved by the Ethics Committee of the College of Medicine & Health Sciences at Sultan Qaboos University.
Results
Table 1 shows the distribution of cases and controls according to the demographic and clinical characteristics of the mothers. The mean age among the cases was 27.8 ± 5.7 years compared to 29.3 ± 5.3 years in the control group (P = 0.004). The average gestational age was 37.6 ± 2.1 weeks and the average weight of the babies was 2,773.3 ± 595.6 g. There were more primiparous women in the case group (33.3%) and more multiparous women in the control group (74.7%). The average number of women with pregnancies complicated by pregnancy-induced HTN and premature rupture of membrane (PROM) was higher among the cases (9.3 and 5.3%, respectively) than the controls (1.7 and 0.3%, respectively; P ≤0.001). Neither the mode of delivery nor the gender of the babies was found to be associated with LBW (P = 0.154 and P = 0.841, respectively).
Table 1:
Distribution of cases and controls according to demographic and clinical characteristics
| Description | Cases n = 150 | Controls n = 300 | P value |
|---|---|---|---|
| Mean maternal age (years ± SD) | 27.8 ± 5.7 | 29.3 ± 5.3 | 0.004 |
| Mean gestational age (weeks ± SD) | 36.2 ± 2.66 | 38.5 ±1.25 | <0.001 |
| Mean birth weight (g ± SD) | 2110.3 ± 367.4 | 3104.9 ± 366.9 | <0.001 |
| Mean BMI (± SD) | 24.4 ± 6.3 | 26.4 ± 6.0 | 0.001 |
| Age groups, n (%) | |||
| <20 years | 9 (6) | 6 (2) | |
| 20–24 years | 36 (24) | 54 (18) | |
| 25–29 years | 54 (36) | 105 (35) | 0.047 |
| 30–34 years | 32 (21.3) | 82 (27.3) | |
| >34 years | 19 (12.7) | 53 (17.4) | |
| Gravidity, n (%) | |||
| Primiparae | 50 (33.3) | 77 (25.7) | 0.088 |
| Multiparae | 100 (66.7) | 223 (74.3) | |
| Gestational age at delivery, n (%) | |||
| Preterm | 41 (27.3) | 19 (6.3) | <0.001 |
| Full term | 109 (72.7) | 281 (93.7) | |
| Past maternal history, n (%) | |||
| Chronic DM | 2 (1.3) | 4 (1.3) | 1.000 |
| Chronic HTN | 3 (2.0) | 6 (2) | 1.000 |
| Hypothyroidism | 2 (1.3) | 3 (1) | 0.750 |
| SCD | 4 (2.7) | 4 (1.3) | 0.313 |
| SLE | 1 (0.7) | 3 (1) | 0.722 |
| Antenatal complication, n (%) | |||
| Preeclampsia | 1 (0.7) | 1 (0.3) | 0.616 |
| PIH | 14 (9.3) | 5 (1.7) | <0.001 |
| GDM | 20 (13.3) | 37 (12.3) | 0.764 |
| PROM | 8 (5.3) | 1 (0.3) | <0.001 |
| Gender of baby, n (%) | |||
| Male | 70 (46.7) | 137 (45.7) | 0.841 |
| Female | 80 (53.3) | 163 (54.3) | |
| Mode of delivery, n (%) | |||
| SVD | 103 (68.7) | 225 (75) | 0.154 |
| CS | 47 (31.3) | 75 (25) | |
SD = standard deviation; BMI = body mass index; DM = diabetes mellitus; HTN = hypertension; SCD = sickle cell disease; SLE = systemic lupus erythematosus; PIH = pregnancy-induced hypertension; GDM = gestational diabetes mellitus; PROM = premature rupture of membrane; SVD = spontaneous vaginal delivery; CS = Caesarean section.
Figure 1 shows the distribution of BMI groups in both cases and controls. The percentage of underweight mothers (BMI <18.5) was higher among the cases compared to the controls (17.3% versus 6%, P <0.001). On the other hand, mothers with normal weight babies were more often overweight or obese compared to mothers with LBW babies. Figure 2 shows the distribution of recommended weight gain groups according to BMI. The percentage of cases with less-than-recommended weight gain was higher among the cases compared to the controls (57.7% versus 33%; P <0.001).
Figure 1:
Distribution of BMI groups among cases and controls.
P value = 0.001.
Figure 2:
Distribution of gestational weight gain among cases and controls.
P value <0.001.
Table 2 presents the adjusted odds ratio (OR) with 95% confidence interval (CI) for BMI groups, using normal BMI as the reference category, and gestational weight gain groups, with recommended gestational weight gain as the reference category. Adjustments were made for the effect of maternal age, gravidity, gestational age at delivery, pregnancy-induced HTN and premature rupture of membrane (PROM). The OR of LBW was 2.27 times higher among underweight mothers compared to 0.83 among overweight and 0.75 among obese mothers. Furthermore, the OR of LBW was 2.67 times higher in women who gained less than the recommended gestational weight compared to women who gained the recommended weight.
Table 2:
Adjusted odds ratio with 95% confidence interval for body mass index and gestational weight gain groups
| Adjusted OR* | Percentage CI | P value | |
|---|---|---|---|
|
BMI groups Normal BMI group used as a reference | |||
| Underweight | 2.27 | 1.09–4.71 | 0.02 |
| Overweight | 0.83 | 0.47–1.47 | 0.53 |
| Obese | 0.75 | 0.38–1.47 | 0.41 |
|
Weight gain Normal weight gain group used as a reference | |||
| Less than recommended | 2.67 | 1.57–4.52 | <0.001 |
| Above recommended | 0.97 | 0.51–1.86 | 0.94 |
Adjusted for maternal age, gravidity, gestational age at delivery, pregnancy induced hypertension, premature rupture of membrane.
OR = odds ratio; CI = confidence interval; BMI = body mass index.
Discussion
This study was the first to examine the relationship between pre-pregnancy BMI, gestational weight gain and LBW babies in Oman. The negative health consequences of a high incidence of LBW babies in a country puts considerable strain on its healthcare resources, facilities and future plans.2 Therefore, many international organisations, such as WHO have expended considerable effort towards improving the global burden of LBW.
This study specifically highlights the effects of pre-pregnancy BMI and gestational weight gain on LBW deliveries. Several maternal factors may contribute to LBW babies. Some of these factors, such as weight, gestational weight gain, smoking and socioeconomic status, are modifiable; however, others, such as age, height and inherited diseases, are not modifiable.
The age of the mother can play both harmful and protective roles in relation to LBW. This study found that most women younger than 20 years gave birth to LBW babies whereas most who were over 35 years gave birth to normal weight babies. This fact has been shown in previous studies in which youth counts as a risk factor for LBW and older age as a protector. The latter may be attributed mainly to the higher parity in older women.13
In the current sample, about 36% of cases and 35% of controls were between 25 and 29 years of age, which demonstrates the fact that the age at which women get married in Oman has increased due to lifestyle changes and increasing levels of education.5
Delivering before the expected due date (preterm <37 weeks) has a significant impact on the weight of the baby and acts as a risk factor for intellectual and physical development in future life. The factors influencing birth weight and prematurity included smoking, HTN (both pre-pregnancy or pregnancy-induced), DM, anaemia, the weight of mothers before pregnancy, preterm PROM, and gestational weight gain.14–16 In this study, it was found that those women who gave birth before 37 weeks were at about a 27.3% higher risk of delivering LBW babies [Table 1]; the major factors influencing this situation were pregnancy-induced HTN and preterm PROM. Even after adjusting for preterm deliveries, there was a significant relationship between maternal weight gain, BMI and LBW in the current study.
Several studies have shown a direct relationship between a mother’s health status before and during pregnancy, and LBW.17–19 In this study, a medical history including all possible diseases that mothers suffered before pregnancy was analysed, but there was no significant difference between the cases and controls. The diseases that mothers may have had during pregnancy, especially pregnancy-induced HTN and PROM, were associated with LBW of newborns. There were no pathologies, such as abnormal cord insertions, in either the case or control groups.
The nutritional status of the mother prior to, during, or after pregnancy directly influences the health status of the fetus. Pre-pregnancy BMI and gestational weight gain have been studied and found to have an important impact on the birth weight and future health of babies.20,21 In the current study, underweight mothers or those who gained less weight than recommended had a two times greater risk of giving birth to LBW babies compared to women with a normal BMI. This finding is consistent with the results of other studies.22–24
As with many other observational studies, this study was prone to errors, including selection bias, misclassification and confounding. First, selection bias may have occurred because women delivering at a tertiary hospital may already have a higher prevalence of risk factors compared to women delivering at other types of hospitals. Therefore, because the controls were selected from the same hospital, such bias may have led to an underestimation of the OR. In addition, the exclusion of mothers who had their first prenatal visit after 13 weeks’ gestation may have introduced a potential selection bias into the estimates. An underestimation of the OR is likely if those who were excluded were likely to be mothers who were underweight. Secondly, because the data was gathered from patient files, information bias or misclassification may have also influenced the study. However, due to the fact that all the exposure data were measured before the delivery of the babies, such misclassification would most likely be non-differential and may not have influenced the estimates. Finally, unmeasured confounders such as socioeconomic factors and the educational levels of mothers may also have affected the findings of this study. Smoking was not adjusted for because it is extremely rare in Oman (only three cases in this study).
Conclusion
This study showed that underweight women, as well as women with less-than-recommended gestational weight gain, were at higher risk of delivering LBW babies. Thus, it is recommended that the issue of appropriate weight gain in relation to pre-pregnancy BMI should be included during prenatal consultations with women of childbearing age as well as pregnant women. Accordingly, more attention needs to be given to nutrition-related consultations offered by health providers during prenatal visits. Further research is needed to explore possible relevant interventions pertaining to improving weight gain during pregnancy.
References
- 1.Kramer MS. Determinants of low birth weight: Methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Children’s Fund (UNICEF) Tracking progress on child and maternal nutrition: a survival and development priority. New York: UNICEF; Nov, 2009. p. 22. From: http://www.childinfo.org/files/Tracking_Progress_on_Child_and_Maternal_Nutrition_EN.pdf Accessed: Feb 2013. [Google Scholar]
- 3.Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;30:313–20. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 4.Choi SK, Park IY, Shin JC. The effects of pre-pregnancy body mass index and gestational weight gain on perinatal outcomes in Korean women: A retrospective cohort study. Reprod Biol Endocrinol. 2011;18:6. doi: 10.1186/1477-7827-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nations Children’s Fund and World Health Organization. Low birthweight: Country, regional and global estimates. UNICEF; New York: 2004. From http://www.childinfo.org/files/low_birthweight_from_EY.pdf Accessed: Aug 2011. [Google Scholar]
- 6.Badshah S, Mason L, McKelvie K, Payne R, Lisboa PJ. Risk factors for low birthweight in the public-hospitals at Peshawar, NWFP-Pakistan. BMC Public Health. 2008;4:197. doi: 10.1186/1471-2458-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valero De Bernabé J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martínez D, et al. Risk factors for low birth weight: A review. Eur J Obstet Gynecol Reprod Biol. 2004;116:3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of National Economy. Statistical Yearly Report. Ministry of National Economy; Muscat, Oman: 2010. [Google Scholar]
- 9.Ministry of Health. Annual report, Health status indicators related to mortality indicators. 2008. From http://www.moh.gov.om/stat/2008/Chapters/CH02Y08.pdf Accessed: Aug 2011.
- 10.Alasfoor D, Traissac P, Gartner A, Delpeuch F. Determinants of persistent underweight among children, aged 6–35 months, after huge economic development and improvements in health services in Oman. J Health Popul Nutr. 2007;25:359–69. [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Global database on body mass index (BMI)/detailed data section. From http://apps.who.int/bmi/index.jsp Accessed: Aug 2011.
- 12.Rasmussen KM, Yaktine AL, editors. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 13.Borga JB, Adair LS. Assessing the net effect young maternal age on birth weight. Am J Hum Biol. 2003;15:733–40. doi: 10.1002/ajhb.10220. [DOI] [PubMed] [Google Scholar]
- 14.Villar J, Belizan J. The relative contribution of prematurity and fetal growth retardation to low birth weight in developing countries. Am J Obstet Gynecol. 1982;143:793–8. doi: 10.1016/0002-9378(82)90012-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox AJ. On the importance and the unimportance of birthweight. Int J Epidemiol. 2001;30:1233–41. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 16.Borkowski W, Mielniczuk H. The influence of social and health factors including pregnancy weight gain rate and pre-pregnancy body mass on low birth weight of the infant. Ginekol Pol. 2008;79:415–21. [PubMed] [Google Scholar]
- 17.Ganesh Kumar S, Harsha Kumar HN, Jayaram S, Kotian MS. Determinants of low birth weight: A case control study in a district hospital in Karnataka. Indian J Pediatr. 2010;77:87–9. doi: 10.1007/s12098-009-0269-9. [DOI] [PubMed] [Google Scholar]
- 18.Alfadhli AM, Hajia AM, Mohammed FAK, Alfadhli HA, El Shazly MK. Incidence and potential risk factors of low birth weight among full term deliveries. Bull Alex Fac Med. 2010;46:152–64. [Google Scholar]
- 19.Rafati S, Borna H, Akhavirad MB, Fallah N. Maternal determinants of giving birth to low-birth-weight neonates. Arch Iran Med. 2005;4:277–81. [Google Scholar]
- 20.Yekta Z, Ayatollahi H, Porali R, Farzin A. The effect of pre-pregnancy body mass index and gestational weight gain on pregnancy outcomes in urban care settings in Urmia, Iran. BMC Pregnancy Childbirth. 2006;20:15. doi: 10.1186/1471-2393-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdanpanahi Z, Forouhari S, Parsanezhad ME. Prepregnancy body mass index and gestational weight gain and their association with some pregnancy outcomes. Iran Red Crescent Med J. 2008;10:326–31. [Google Scholar]
- 22.Ronnenberg AG, Wang X, Xing H, Chen C, Chen D, Guang W, et al. Low preconception body mass index is associated with birth outcome in a prospective cohort of Chinese women. J Nutr. 2003;133:3449–55. doi: 10.1093/jn/133.11.3449. [DOI] [PubMed] [Google Scholar]
- 23.Han Z, Mulla S, Beyene J, Liao G, McDonald SD, Knowledge Synthesis Group Maternal underweight and the risk of preterm birth and low birth weight. Int J Epidemiol. 2011;40:65–101. doi: 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- 24.Frederick IO, Williams MA, Sales AE, Martin DP, Killien M. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Matern Child Health J. 2008;12:557–67. doi: 10.1007/s10995-007-0276-2. [DOI] [PubMed] [Google Scholar]