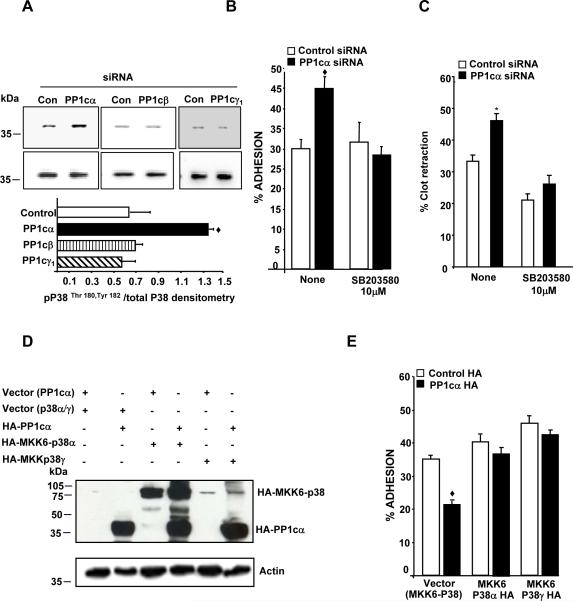
Figure 5. PP1cα negatively regulates αIIbβ3 adhesion by suppressing the p38 signaling pathway.
(A) Lysate from the 293 cells treated with either control, PP1c (α, β, γ1) siRNAs were immunoblotted with anti-phospho-Thr180, Tyr182 p38 (pp38) and p38 antibodies. Densitometric quantification of the activation of p38 was expressed as pp38/total p38 from 2-3 experiments in arbitrary units. The increased P38 activation in PP1cα depleted cells compared to the control siRNA treated cells was significant at ◆p=0.04. Effect of the water soluble p38 inhibitor on the increased fibrinogen adhesion (B) and clot retraction (C) displayed by the PP1cα depleted cells. Compared to control siRNA, the increased adhesion of PP1cα depleted cells at 30 minutes and the increased clot retraction at 60 minutes were significant at ◆p=0.001 and p=0.03. Treatment with SB203580 abolished the increased adhesion and clot retraction differences p=0.618 and p=.138 n=4-5. (D) Expression of PP1cα with either MKK6-P38α or MKK6-p38γ fusion proteins detected by immunoblotting with anti-HA antibody in 293 αIIbβ3 cells overexpressing the appropriate constructs. The blot is a representative of 2-3 experiments. (E) Effect of MKK6-p38α and MKK6-p38γ on the decreased adhesion of PP1cα overexpressing cells to fibrinogen at 15 minutes. Compared to the control HA, the decreased adhesion of PP1cα HA overexpressing cells were significant at ◆p<0.001. These differences were lost in cells expressing MKK6-p38α (p>0.05) or MKK6-p38γ (p>0.0.1) *p=0.006. n=4.