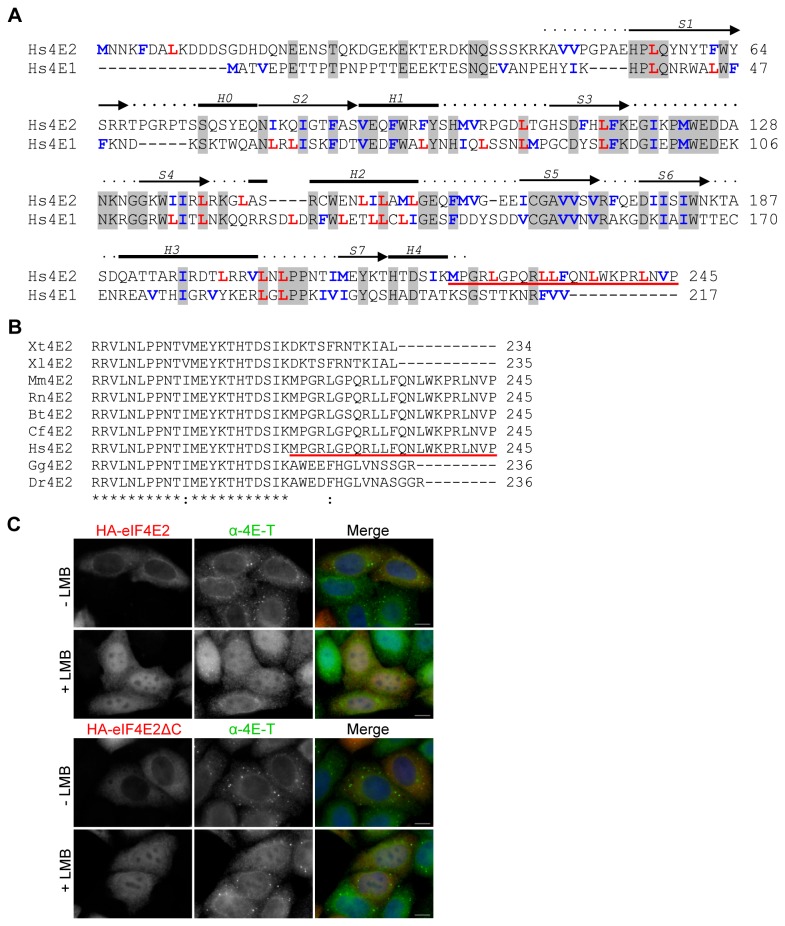
Figure 8. The leucine-rich C-terminus of eIF4E2 does not act as a NES.
A. Sequence alignment of human eIF4E1 and eIF4E2 performed with ClustalW2. Secondary structural elements of α-helices (H0-H4) and β-strands (S1-S7) were shown above the alignment according to the crystal structure of human eIF4E2 in complex with m7 GTP (PDB id: 2JGB) [15]. Identities are shaded in grey. With blue and red are marked hydrophobic residues that typically form the NES. The leucine-rich C-terminus of eIF4E2 is underlined in red. B. ClustalW alignment of the C-terminal regions of indicated eIF4E2 proteins. C. HeLa cells transfected with HA-eIF4E2 full-length protein or HA-eIF4E2ΔC (truncated at residue 222) were treated with LMB or methanol (-) for 5 hrs and immunostained with HA and 4E-T antibodies as indicated. Cells were also stained with DAPI. Scale bar, 10 µm.