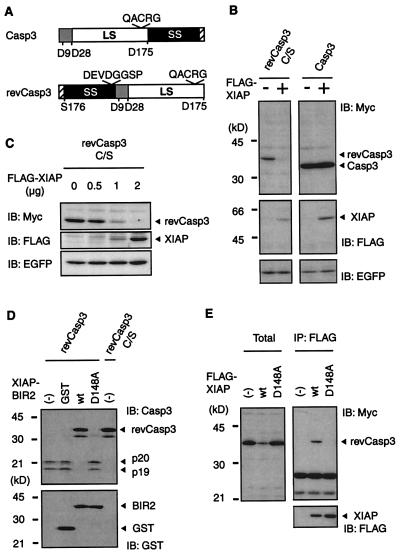
Figure 1.
Regulation of caspase-3 protein expression by XIAP. (A) Schematic representation of the constitutively active caspases used in this study. The procaspase-3 and the constitutively active caspases-3 (revCasp3) are indicated. Locations of the large subunit (LS), small subunit (SS), and prodomain, of each caspase, are illustrated by the white box, black box, and gray box, respectively. Numbers indicate their amino acid position. The cysteine residue of their active site is shown by QACRG. We also constructed inactive mutant of the revCasp3 by substituting its cysteine residue with serine. The hatched boxes represent a Myc tag. (B) Active-form caspase-3 protein expression is specifically decreased in the presence of wild-type XIAP. 293T cells were cotransfected with a pcDNA3 expression vector containing cDNAs (0.5 μg Myc-revCasp3 C/S; 0.5 μg Procaspase-3-Myc; 2 μg, FLAG-XIAP) as indicated, and pEGFP (0.25 μg). The total amount of plasmid was adjusted to 2.75 μg with pcDNA3. Forty-eight hours after transfection, cell lysates were subjected to immunoblot (IB) analysis with anti-Myc, anti-FLAG, or anti-EGFP antibody as indicated. (Bottom) Probes with anti-EGFP antibody verify equivalent transfection efficiencies. (C) 293 cells were cotransfected with 0.25 μg of Myc-revCasp3 C/S, 0.25 μg of pEGFP, and increasing amounts of the XIAP expression plasmids, as indicated. The total amount of plasmid was adjusted to 2.5 μg with pcDNA3. (Bottom) Equivalent transfection efficiencies. (D) The inhibitory effect of wild-type or mutant XIAP on the autoprocessing of revCasp3 in an in vitro translation reaction. RevCasp3 or active site Cys to Ala mutant in pcDNA3-myc constructs were in vitro-translated in the absence or presence of 100 nM of GST-tagged recombinant XIAP-BIR2 proteins. The translation products were analyzed by immunoblot with anti-caspase-3 antibody (Upper). (Lower) The equivalent amounts of GST-proteins were used. (E) The association of XIAP with caspase-3 is essential for the reduction in caspase-3 protein expression. Cell lysates from 293T cells cotransfected with the indicated expression vectors were subjected to immunoblot analysis with anti-Myc (Left). The conditions of transfection were similar to those indicated in B. The same lysates were immunoprecipitated with anti-FLAG antibody followed by probing with anti-Myc (Right) and anti-FLAG antibody (Lower).