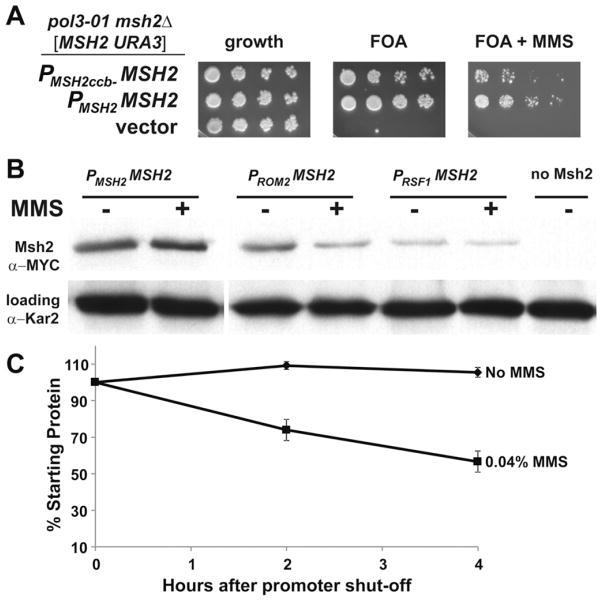
Fig. 5.
The expression of MSH2 in response to MMS is controlled at the transcriptional and post-transcriptional level. (A) Scrambling of cell-cycle elements upstream of MSH2 results reduced viability in response to MMS in a strain in which mismatch repair is essential. AGY1057, an msh2Δ pol3-01 strain kept alive by an MSH2-MYC URA3 plasmid [MSH2-MYC::KanMX6 URA3] was transformed with additional plasmids expressing: MSH2 expressed from its own promoter (PMSH2 -MSH2), MSH2 expressed from its promoter where the cell-cycle boxes are scrambled (PMSH2ccb− -MSH2), or no MSH2 (vector). Two-fold serial dilutions of exponentially growing cells were spotted onto plates containing no drug selection as a control for growth (growth), 5-FOA plates (FOA), or 5-FOA plates with 0.04% MMS (FOA + MMS). 5-FOA selects for cells that were able to lose the covering MSH2 URA3 plasmid. (B) Constitutive expression of Msh2 causes a decrease in Msh2 protein levels in response to MMS. Exponentially growing strains expressing MSH2 with a C-terminal MYC epitope tag driven from either the wild-type MSH2 promoter (PMSH2 -MSH2, strain AGY665) or the constitutive promoter upstream of ROM2 (PROM2 -MSH2, strain AGY1055), or RSF1 (PRSF1 -MSH2, strain AGY1056) were grown in the absence (−) or presence (+) of MMS for 1.5 hours and prepared for immunoblot analysis. A strain expressing an untagged MSH2 (no Msh2, strain AGY220) was included as a negative control. Protein extracts were analyzed by immunoblotting with α-MYC to detect Msh2 or with α-Kar2 as a loading control. (C) MMS increases the turnover of Msh2. A yeast msh2Δ strain (AGY75) harboring a plasmid with the GAL10 promoter fused to MSH2 (pGAL-MSH2) was used to permit controlled expression of Msh2. For the experiment, cells were grown to exponential phase in 2% galactose containing medium. The culture was split and glucose (2%) was added to each culture to repress synthesis of MSH2. One culture was also exposed to MMS (0.04%). At time intervals of 0 h, 2 h, and 4.0 h after repression of synthesis, ~3 × 107 cells were processed for immunoblotting. Protein extracts were subjected to chemiluminescence immunoblotting methods to detect Msh2 variant and a loading control. The data were normalized for growth (see Section 2) and are expressed as a percentage of the zero time point (% Starting Protein).