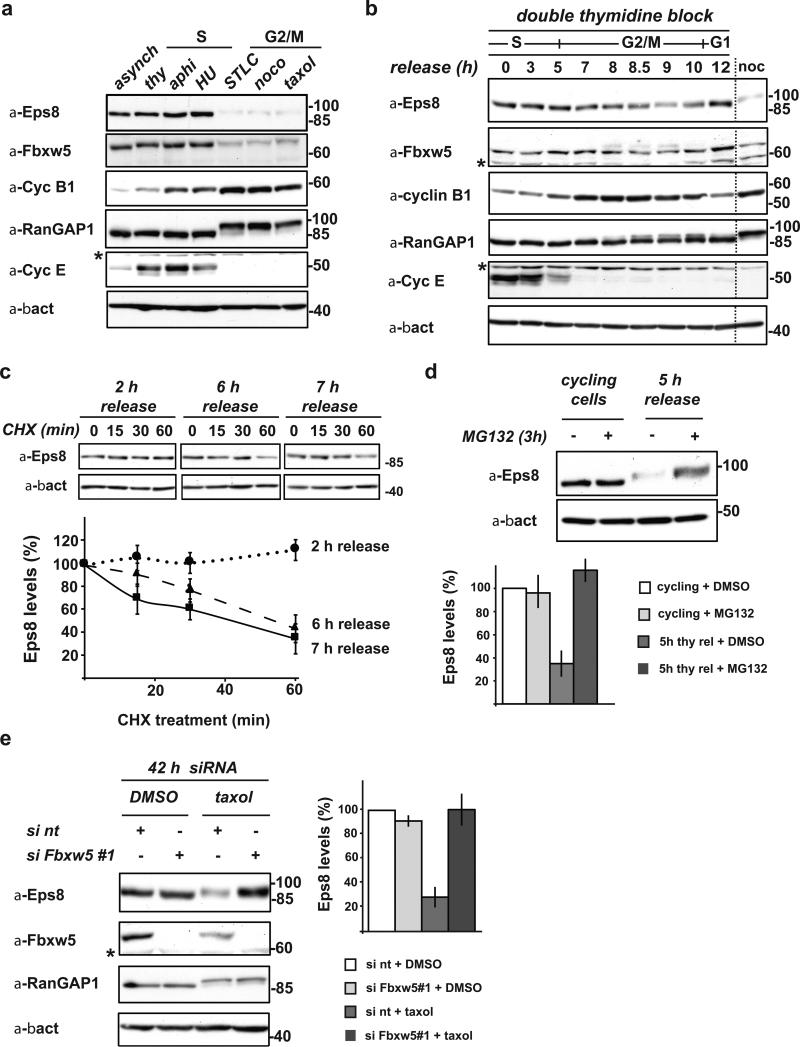
FIGURE 1. Eps8 is a cell cycle regulated protein that undergoes Fbxw5-mediated proteasomal degradation specifically during G2/M.
(a) Immunoblot analysis of exponentially growing (cycling), thymidine-, aphidocolin-, or hydroxurea-arrested (S-Phase), and STLC, nocodazole, or taxol-arrested HeLa cells (G2/M) with indicated antibodies.
(b) HeLa cells were synchronized by a standard double thymidine block/release protocol. Samples were taken after indicated time periods and lysates analyzed by immunoblotting. For comparison, lysate of nocodazole-arrested cells (as in (a)) were loaded (noc).
(c) HeLa cells were synchronized by a standard double thymidine block and released. After indicated time periods, cycloheximide (CHX) was added. Samples were taken at indicated time points after CHX addition, analyzed by immunoblotting. Eps8 levels were quantified relative to β-actin levels and plotted against time after addition of CHX. Eps8 levels at 0 min CHX were set to 100% (mean, +/- s.e.m. of three independent experiments).
(d) Exponentially growing HeLa cells and cells released from a double thymidine block for 5 h were incubated with 20 μM MG132 or mock-treated with DMSO for 3 h. Eps8 levels were anaylzed by immunoblotting (top panel) and quantified relative to β-actin levels (bottom panel); relative Eps8 levels of mock-treated cells set to 100 (mean, +/- s.e.m. of three independent experiments).
(e) Immunoblot analysis of HeLa cells transfected with non-targeting siRNA (si nt) or siFbxw5#1 for 42 h with indicated antibodies. 24 h after siRNA treatment, cells were treated with taxol or mock-treated with DMSO for 18 h. Eps8 levels were quantified relative to β-actin levels; relative Eps8 levels of control cells (si nt + DMSO) were set to 100 % (mean, +/- s.e.m. of three independent experiments). * cross-reactive band