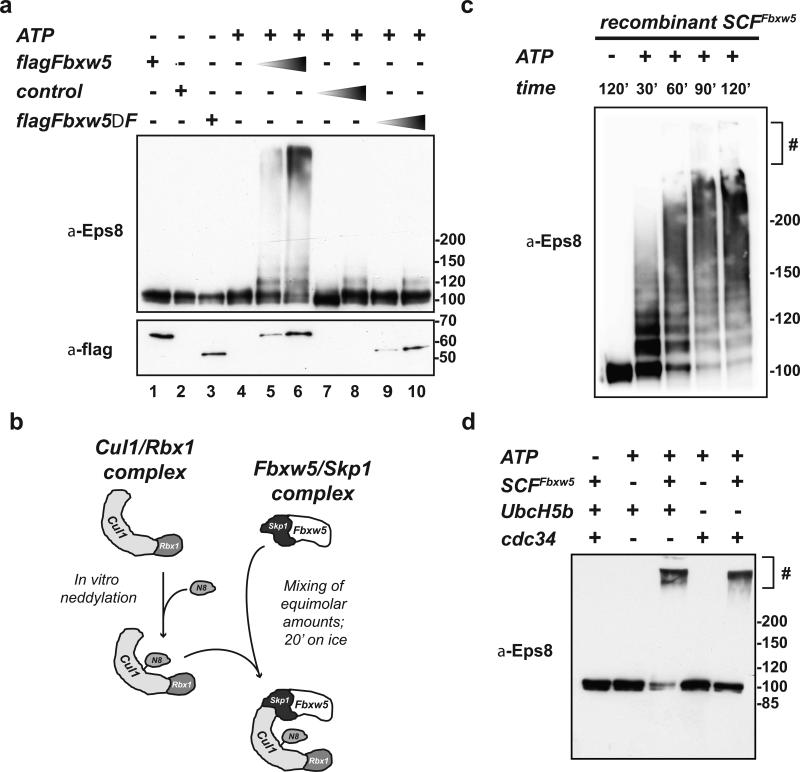
FIGURE 2. Eps8 is a substrate of SCFFbxw5 in vitro.
(a) In vitro ubiquitylation reaction of His-Eps8 (0.2 μM) with 75 μM His-Ubiquitin, 170 nM Ube1, 1 μM UbcH5b, and 5 mM ATP in the absence or presence of different amounts of control (= flag-IPs from non-transfected cells), flag-Fbxw5, and flag-Fbxw5ΔFbox immunoprecipitates at 30°C for 120 min.
(b) For SCFFbxw5 reconstitution, mouse Fbxw5/Skp1 complexes were purified from bacteria (see material and methods) and Cul1/Rbx1 complexes were purified from E.coli using a ”split and co-express“ approach and in vitro neddylated as previously described31, 32. For complex formation, both sub-complexes were mixed in equimolar amounts and incubated on ice for 20 min.
(c) Time course of SCFFbxw5-dependent Eps8 ubiquitylation. 0.2 μM His-Eps8 was incubated with 75 μM His-Ubiquitin, 170 nM Ube1, 0.5 μM UbcH5b, 50 nM SCFFbxw5, and 5 mM ATP at 30°C. Reactions were stopped at indicated time points, and analyzed by immunoblotting. # stacking gel
(d) In vitro ubiquitylation of His-Eps8 (0.2 μM) with 75 μM His-Ubiquitin, 170 nM Ube1, 1 μM UbcH5b or Cdc34, 5 mM ATP in the absence and presence of 150 nM reconstituted SCFFbxw5 at 30°C for 90 min.