Abstract
The elusive nature of Trichomonas vaginalis, the most common, non-viral, sexually transmitted pathogen has hampered our knowledge of its significance for human health for over 150 years. The combination of epidemiology, molecular cell biology, immunology and more recently genomics and other allied omics data, are all contributing at shedding new light onto what is increasingly recognised as a significant human pathogen leading to important health sequelae due to multifaceted interactions with its human host, the human microbiota, bacterial pathogens and viruses. The integrations of these various data are contributing in important ways to refining our understanding of the parasite pathobiology and virulent factors. Indeed, it is increasingly recognised that to rationalise the development of effective prophylactic and therapeutic treatments for human pathogens it is important to integrate the broadest possible spectrum of human-microbial-parasite-virus interactions in relation to qualitative and quantitative variations in the human innate and adaptive defence responses. This short review aims at providing an integrative overview of T vaginalis virulent factors by taking into account the importance of the human-microbiota-parasite-virus interplay in human health. It also highlights selected cellular characteristics of the parasite often overlooked in the biological and medical literature.
Keywords: ADHERENCE, HIV, TRICHOMONAS, VAGINAL MICROBIOLOGY, PARASITOLOGY
Introduction
Ever since its discovery in 1836 by the French medical doctor and microbiologist, Alfred F. Donné, the sexually transmitted obligate extracellular mucosal parasite Trichomonas vaginalis (TV) has progressively grown, rather sluggishly, from a perceived insignificant commensal to an important pathogen, inducing significant health sequelae in both men and women, and with adverse pregnancy outcomes.1 2 Some of the most recent developments in our understanding of TV pathobiology include its epidemiological association with HIV over and above high-risk members of the population in terms of exposure to sexually transmitted infections (STI),2 3 and a positive association with aggressive prostate cancers.4 The dramatic impact on human health of the HIV pandemic and resource-limiting conditions experienced by hundreds of millions of people worldwide have combined in highlighting the pathogenic significance of this common human parasite—one that is facilitating the spread of HIV and is associated with a number of reproductive complications including sterility, preterm birth, underweight newborn babies and cervical cancer.1–3 Significantly, TV infects a large fraction of individuals, in particular men, without overt symptoms, complicating its diagnosis and control.3 5 Furthermore, and particularly relevant in the context of a discussion on TV virulent factors, the parasite's elusive nature also renders the characterisation of the molecular and cellular basis of its pathobiology less straightforward, as highlighted for similarly elusive bacterial pathogens.6 Indeed, there is growing recognition that the outcome of human–symbiont interactions (encompassing mutualists, commensals and parasite/pathogens) cannot be fully understood without considering the host immunological status and host-associated microbiota.7 There is a need to integrate the full range of human-microbe interactions in all its diversity to comprehend their different contributions and influence on each other, and how these impact both positively and negatively on human health.7 These considerations also underscore the limitations of the reductive approach implied by the original four Koch postulates and their molecular children, used to characterise pathogens and their virulence factors.6 Incorporating the host response to exposures to microbes is now considered to be an integral part of defining pathogens and their virulence factors.6 8 Indeed, these issues are increasingly recognised by the research community working on TV, as illustrated in a number of recent reviews.4 9–12 Hence, an integrative overview of TV virulence factors is presented here, acknowledging the complex host-microbiota-parasite-virus interplay in influencing the outcomes of human-TV interactions.
Cellular and molecular basis of TV virulence
A number of recent reviews have discussed various aspects of well-characterised and more speculative virulence factors of TV.10–13 Only a selection of TV virulence factors will be considered here and integrated with some of the most recent data on TV molecular cell biology and human innate and adaptive responses to TV. Some important gaps in our knowledge of TV pathobiology will also be highlighted. The evolutionary origin of the best-studied organelle of the parasite, the hydrogenosome, and the roles of hydrogenosomal enzymes in the parasite's physiology and as a drug target were recently reviewed10 14 and are not covered here.
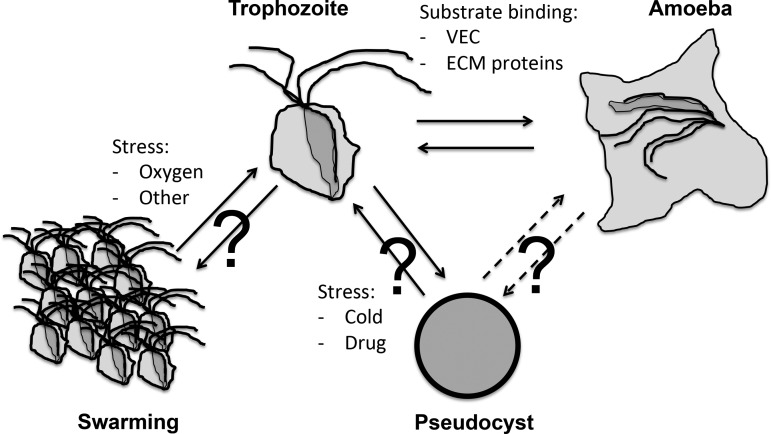
TV is a flagellated microbial eukaryote known to exist in several cellular forms (figure 1). The two best characterised forms are the trophozoite, a free-swimming, flagellated, pear-like cell, and an amoeboid form, with a pancake shape characterised by an important increase in surface contact; this is rapidly induced upon trophozoite contact in vivo with epithelial cells from the vagina, cervix, urethra, prostate and extracellular matrix (ECM) proteins (figure 1).1 11 12 Trophozoites are typically considered as the infective form. A third cellular form called pseudocysts can be induced in vitro upon exposure of trophozoites to cold and other stressors.15 However, the significance of this form during the infection process is currently unknown.1 Data for the related bovine parasite Tritrichomonas foetus, which leads to various reproductive complications including abortion and sterility, suggest that pseudocysts are highly relevant corresponding to aggressive forms facilitating transmission to, and the initial colonisation of, a new host.16 17 Additionally, aggregates of parasites are also known to form (figure 1). Very little is known concerning the triggers and cellular signalling that orchestrate TV differentiations into these different forms.11 Identifying specific molecular markers for the different TV cellular forms and aggregates will be an essential prerequisite to investigate their relevance during the infection process. The draft genome sequence and its annotation represent an invaluable resource to investigate the molecular cell biology of TV by providing specific molecular leads and allowing comparative transcriptomics and proteomics investigations.10 18 An initial study of the phospho-proteome of TV trophozoites, amoeba and pseudocysts grown in vitro suggested differential protein phosphorylation profiles, consistent with specific signalling mechanisms occurring in the different cellular forms of the parasite that are induced upon specific environmental triggers.19
Figure 1.
Various cellular forms of Trichomonas vaginalis (TV). The TV trophozoite is characterised with four anterior and a fifth recurrent flagella. Upon contact with human tissue, TV can rapidly differentiate into an amoeboid form. In vitro trophozoites can differentiate into pseudocysts upon exposure to cold. Pseudocysts are spherical cells without visible flagella (they are internalised), they can divide and redifferentiate into trophozoites.15 It is not known if amoeboid form can differentiate into pseudocysts (?). Trophozoites are also known to form large cellular aggregates, in a process called swarming. Such groups of cells are thought to have stronger binding and cytotoxicity properties towards human tissues, and could also represent a form of defensive reaction to the host immune responses.21.
Clinical isolates of TV have been accumulated over the years from many regions of the world.20 21 Comparisons of their capacity to bind and kill human (and other species) cell lines in vitro have demonstrated important variations between TV isolates.13 21 Accordingly, these capacities are considered important virulent traits for TV.11–13 One of the currently best-characterised adhesins mediating parasite binding to host tissue are TV lipoglycans (TvLG), the most abundant surface molecules of the parasite.13 Investigating TvLG also led to the identification of galectin-1, the only identified human receptor for TV13 so far. TvLGs are also known to modulate inflammatory responses of epithelial cells and macrophages.13 A proteomics survey of TV surface proteins identified a total of 411 proteins,22 confirming a number of in silico-predicted surface proteins, and also a host of novel and important candidate virulent factors.11 13 22 By contrasting strains with low versus high level of adhesion to vaginal epithelial cells (VECs) in vitro, 11 proteins were shown to be more abundant on the cell surface of the highly adhering strains.22 These include proteins annotated as hypotheticals—which have no sequence similarity to any other proteins in protein databases. A high level of expression of two of these hypothetical proteins increased the binding to VECs of a poorly binding TV strain.22 Other novel candidate cell surface virulence factors identified in this proteomics survey included three tetraspanins, which are membrane proteins involved in signalling modulating adhesion, motility and tissue invasion in other systems; all these are key processes underlying TV pathobiology.13 The characterisation of one TV tetraspanin demonstrated that its expression and cellular localisation was modulated upon TV binding to VEC, and that it plays a role in regulating migration of the parasite through a surrogate ECM gel, strongly supporting tetraspanins as important TV virulence factors.23 These studies highlight how little we know about key aspects of TV biology, and how genomics and allied omics provide important tools to investigate host-parasite interactions.10 Related proteomics studies on TV are reviewed elsewhere.10–13
During the infection process, TV actively phagocytoses human cells, bacteria and fungi to obtain nutrients.11 12 Similarly, receptor-mediated endocytosis by TV is also considered important in order to internalise nutrients and iron, and to neutralise host defence proteins.11 12 However, there are currently no functionally characterised TV genes encoding surface proteins mediating the specific binding process underlying phagocytosis or endocytosis.11 12 Bioinformatic analyses of candidate transmembrane proteins have identified a number of cytoplasmic tails possessing classic signals for endocytosis, some of which were supported by proteomics data (table 1).11 13 In silico identification of candidate genes regulating membrane trafficking have also identified a surprisingly large repertoire of proteins, with some gene families unexpectedly larger than those encoding human homologues (eg, ∼300 TvRab vs ∼70 HsRab GTPases), further suggesting that phagocytosis and endocytosis are important processes for the parasite and represent promising targets for interfering with parasite virulence.11 12 18
Table 1.
Example of Trichomonas vaginalis (TV) candidate transmembrane proteins potentially mediating endocytosis*
| Protein family | RefSeq accession | Protein length | [FY]NPX[FY] motif | Acidic cluster |
|---|---|---|---|---|
| BspA | XP_001308117 | 735 | NENPIF | DDPFAADFMDS |
| BspA | XP_001584393 | 1092 | FTNPLF | DDPFASDFDDP |
| BspA | XP_001321489 | 600 | YDNPFF | DDPFASDFQND |
| BspA | XP_001321493 | 582 | YDNPFF | DDPFASDFQND |
| BspA | XP_001321496 | 594 | FDNPLF | DDPFASDFQNE |
| BspA | XP_001321499 | 447 | FDNPLF | DDPFASDFQND |
| BspA | XP_001321508 | 733 | FDNPLY | DDPFAKDFQNE |
| BspA | XP_001323867 | 405 | LDNPLF | DDPFAEDFEEK |
| BspA | XP_001328761 | 549 | FSNPLF | EDPFANDFEEG |
| BspA | XP_001328763 | 1142 | YINPLF | DDPFASDFEDH |
| BspA | XP_001328767 | 834 | FTNPLF | DDPFASDFEEH |
| BspA | XP_001312366 | 703 | ADNPIF | DDPFAQDFNDD |
| Pmp | XP_001579443 | 593 | NDNPLW | DDPFRKDFEEE |
| Pmp | XP_001313554 | 742 | DENPLW | DDPFRNDFEEV |
| Pmp† | XP_001325298 | 624 | NDNPLW | DDPFKKDFEEE |
| Pmp | XP_001306144 | 569 | IDNPLW | SDPFRTEFEEK |
| Pmp | XP_001582856 | 712 | NDNPLW | DDPFKNDFEED |
| Pmp† | XP_001309734 | 812 | NDNPLW | DDPFKNDFEED |
| Pmp | XP_001328616 | 605 | YTNPLW | DDPFVDDFQEQ |
| Pmp | XP_001581046 | 680 | NDNPLW | DDPFKNDFEEE |
| Pmp | XP_001325882 | 803 | NDNPLW | EDPFKDDFNEI |
| Pmp | XP_001325146 | 593 | QENPLW | DDPFKGDFQEQ |
| Pmp | XP_001320330 | 526 | NVNPLF | DDPFRQDFEEK |
| Pmp | XP_001320335 | 1819 | NDNPLW | DDPFNNDFEED |
| Pmp† | XP_001299221 | 652 | NENPLW | ENPFLNDFEED |
*Entries with at least one inferred transmembrane domain from two distinct TV protein families (BspA: Bacteroides surface protein A-like extracellular domain and Pmp: Chlamydia polymorphic membrane protein-like extracellular domain) but with a shared cytoplasmic tails possessing two motifs ([FY]NPX[FY] and acidic clusters) representing potentially signals for endocytosis—for details see ref. 11.
†Entries supported by cell surface proteomics.22
TV interactions with human-associated bacteria and viruses
The phagocytosis of human microbiota by TV, including bacteria and fungal cells, has the potential to induce dysbiosis (imbalanced microbial community7) in the human microbiota, for example, as observed during bacterial vaginosis (BV). Consistent with this possibility, a correlation between the presence of TV and a vaginal microbiota characterised with low abundance of protective lactobacilli and higher proportions of Mycoplasma, Prevotella and other bacteria typically observed in BV, has been observed.9 Future work will be required to establish temporality and causality between BV and TV, and their contribution (possibly synergistic) to increase host susceptibility to HIV.9 In addition, antibiotics can significantly disturb the host-microbiota-viruses balances, and are also increasingly recognised to contribute to various disease conditions.7 Hence, the potential negative impact of the treatment of TV with metronidazole/tinidazole should also be considered in this global framework of human–microbes interactions.
TV has been shown to form a symbiosis with Mycoplasma hominis in in vitro cultures, and Mycoplasma cells carried by TV could infect human cells, suggesting that TV could be a Trojan horse for the bacterium.24 Intriguingly, coinfections with TV-Mycoplasma were shown to consume larger amounts of free arginine than TV alone, which could contribute to reducing nitric oxide production by macrophages through depletion of free arginine in the vagina, thus potentially interfering with an important host defence mechanism.25
Another likely consequence of TV actively feeding on host microbiota, is the acquisition of a number of bacterial genes through lateral gene transfer (LGT). Of particular interest is an almost complete set of enzymes of bacterial origin capable of degrading glycans, and mostly acquired from members of the bacteroidetes,26 one of the most abundant bacterial lineage of mucosal microbiota and known to encode a rich set of glycan-targeting enzymes27 (table 2). These TV enzymes of bacterial origins are thought to play important roles in degrading mucins and other human glycans, including those from the glycocalyx of epithelial cells, which represent the initial barrier the parasite needs to deal with to be able to colonise the host mucosal surfaces.28 Glycan degradation is also likely to provide an important source of energy for the parasite.
Table 2.
List of enzymes involved in glycan metabolism derived from bacterial lateral gene transfers (LGTs)*
| EC number† | Enzyme name | RefSeq accession | Nearest neighbour in phylogeny |
|---|---|---|---|
| 3.2.1.18 | Exo-α-sialidase | XP_001319692 | Bacteroidetes |
| 3.2.1.23 | β-galactosidase | XP_001581038 | Bacteroidetes |
| 3.2.1.52 | β-N-acetylhexosaminidase | XP_001329989 | Bacteroidetes |
| 3.2.1.24 | α-mannosidase | XP_001579222 | Prokaryotes |
| 3.2.1.25 | β-mannosidase | XP_001322689 | Proteobacteria |
| 3.2.1.51 | α-fucosidase | XP_001316088 | Bacteria |
| 4.1.3.3 | N-acetylneuraminate lyase | XP_001323296 | Pasteurellaceae (γ-proteobacteria) |
| 5.1.3.8 | Acylglucosamine 2-epimerase | XP_001308218 | Bacteroidetes |
| 3.2.1.45 | Glucosylceramidase | XP_001279673 | Bacteroidetes |
*For details about inference of LGT methodology and gene and enzyme characteristics see ref. 26. Note that some of the listed enzymes could target O-glycans, N-glycans and/or gangliosides.
†EC, Enzyme commission number.
Tissue damage and inflammatory responses due to TV infections are thought to facilitate HIV entry and, in TV-HIV dually infected patients, to stimulate HIV production.1 Additionally, TV can also internalise human viruses through endocytosis.11 Viruses carried by TV could contribute to their transmission to a new host, as viruses internalised by TV were shown to be infectious for human cells for 2–6 days. The viruses are either released upon parasite death or secreted from TV-loaded viruses through the recycling route of the endocytic pathway.11 However, there is no evidence that any human virus can replicate in TV. Hence, as suggested for Mycoplasma, TV could be a Trojan horse for HIV and possibly other viruses.
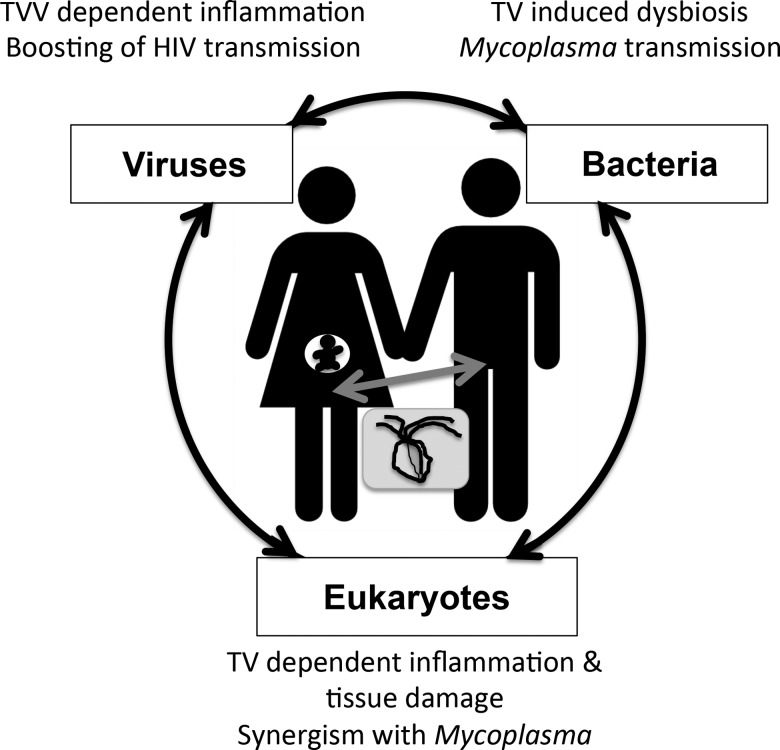
These different considerations clearly illustrate the importance of studying a pathogen in the broader context of human–microbes interactions (figure 2).7
Figure 2.
Interactions between Trichomonas vaginalis (TV), viruses, microbiota and the human host and their impact on human health. The diagram illustrates a TV-infected pregnant woman and her partner in the global context of human–microbe interactions.7 TV infections can affect the health status of both the adults and the embryo (eg, preterm birth and HIV transmission in utero).1 3 Rather then single/linear human–pathogen interactions, it is the complex network of interactions between viruses-bacteria-eukaryotes and the human host that should be considered when investigating disease conditions.7
TV viruses
Adding to its capacity to physically interact with human viruses and facilitate HIV transmission through damaging mucosal surfaces, TV can be infected with four dsRNA viruses (TVV) members of the Totiviridae, thought to be transmitted mainly vertically.29 Various data suggest, collectively, that TVV modulate the parasite virulence by influencing TV gene expression; however, additional studies are required to firmly establish this fascinating possibility.29 Two recent developments are of particular interest in relation to a potential role of TVV in TV pathobiology. A study investigating TV genetic diversity and population structure identified two major genotypes for the parasite (TV1 and TV2) with distinct properties.20 TV1 isolates are more sensitive to metronidazole, and are also significantly more likely to be infected with TVV than TV2, in a ratio ∼3:1.20 In another study investigating the potential role of TVV in modulating the human innate immune system, it was established that TVV are sensed by the human toll-like receptor 3 (TLR3), and that this induces an innate response, including proinflammatory cascades, previously implicated in preterm birth and HIV-1 susceptibility.30 These new aspects of TV pathobiology should be taken into account in future research on TV pathobiology, and influence, for example, how clinical samples are processed for research and, possibly, diagnostics. For instance, to allow quantification of TVV, processing of both total DNA (to investigate TV, bacteria, etc…) and RNA will be required.
Conclusion
The aim of this short overview on TV virulence factors was to illustrate specific aspects of the intricate and complex interactions taking place between the parasite and its host. The complex interplay between the human urogenital tracts, TV and other human pathogens, human microbiota and TVV emphasise the importance of integrating the broadest possible spectrum of human–microbes interactions when studying human–TV interactions. The challenge will be to be able to take into consideration these dynamic host–microbes interactions to guide future research projects on TV molecular cell biology, immunology and epidemiology to eventually produce more refined and effective treatment protocols for STIs. The paper by Fiori et al,31 further illustrates the synergistic interactions between TV and Mycoplasma.
Key messages.
The pathobiology of Trichomonas vaginalis (TV) is multifaceted involving direct and indirect interactions with host tissue, bacteria, human viruses and Trichomonas viruses.
The genome sequence data of TV is being exploited to facilitate the study of the molecular basis of the parasite pathobiology.
The immune response to TV is still poorly understood. and it will be essential to study its variations to rationalise the outcome of host–parasite interactions.
Acknowledgments
I thank Didier Ndeh for critical reading of an early draft of the manuscript. My apologies to the authors whose work could not be cited due to length restrictions.
Footnotes
Funding: Past support from the Wellcome Trust for my research on Trichomonas vaginalis is greatly acknowledged.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Hobbs MM, Sena AC, Swygard H, et al. Trichomonas vaginalis and trichomoniasis. In: Holmes K, Holmes KK, Sparling PF, et al., eds. Sexually transmitted disease. 4th edn, New York: McGraw-Hill, 2008:771–93 [Google Scholar]
- 2.McClelland RS. Trichomonas vaginalis infection: can we afford to do nothing? J Infect Dis 2008;197:487–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston VJ, Mabey DC. Global epidemiology and control of Trichomonas vaginalis. Curr Opin Infect Dis 2008;21:56–64 [DOI] [PubMed] [Google Scholar]
- 4.Sutcliffe S, Neace C, Magnuson NS, et al. Trichomonosis, a common curable STI, and prostate carcinogenesis—a proposed molecular mechanism. PLoS Pathog 2012;8:e1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Pol B. Trichomonas vaginalis infection: the most prevalent nonviral sexually transmitted infection receives the least public health attention. Clin Infect Dis 2007;44:23–5 [DOI] [PubMed] [Google Scholar]
- 6.Falkow S. Molecular Koch's postulates applied to bacterial pathogenicity–a personal recollection 15 years later. Nat Rev Microbiol 2004;2:67–72 [DOI] [PubMed] [Google Scholar]
- 7.Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell 2012;148:1258–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall A, Pirofski LA. Virulence factors and their mechanisms of action: the view from a damage-response framework. J Water Health 2009;7(Suppl 1 Special issue on Trichomonas vaginalis):S2–18 [DOI] [PubMed] [Google Scholar]
- 9.Brotman RM, Bradford LL, Conrad M, et al. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis 2012;39:807–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad MD, Bradic M, Warring SD, et al. Getting trichy: tools and approaches to interrogating Trichomonas vaginalis in a post-genome world. Trends Parasitol 2013;29:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirt RP, de Miguel N, Nakjang S, et al. Trichomonas vaginalis pathobiology new insights from the genome sequence. Adv Parasitol 2011;77:87–140 [DOI] [PubMed] [Google Scholar]
- 12.Figueroa-Angulo EE, Rendon-Gandarilla FJ, Puente-Rivera J, et al. The effects of environmental factors on the virulence of Trichomonas vaginalis. Microbes Infect 2012;14:1411–27 [DOI] [PubMed] [Google Scholar]
- 13.Ryan CM, de Miguel N, Johnson PJ. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem 2011;51:161–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller M, Mentel M, van Hellemond JJ, et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol Mol Biol Rev 2012;76:444–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira-Neves A, Ribeiro KC, Benchimol M. Pseudocysts in trichomonads–new insights. Protist 2003;154:313–29 [DOI] [PubMed] [Google Scholar]
- 16.Pereira-Neves A, Nascimento LF, Benchimol M. Cytotoxic effects exerted by Tritrichomonas foetus pseudocysts. Protist 2012;163:529–43 [DOI] [PubMed] [Google Scholar]
- 17.Pereira-Neves A, Campero CM, Martinez A, et al. Identification of Tritrichomonas foetus pseudocysts in fresh preputial secretion samples from bulls. Vet Parasitol 2011;175:1–8 [DOI] [PubMed] [Google Scholar]
- 18.Carlton JM, Malik SB, Sullivan SA, et al. The genome of Trichomonas vaginalis. In: Clark CG, Johnson PJ, Adam RD. Anaerobic parasitic protozoa: genomics and molecular biology. Norfolk, UK: Caister Academic Press, 2010:45–77 [Google Scholar]
- 19.Yeh YM, Huang KY, Richie Gan RC, et al. Phosphoproteome profiling of the sexually transmitted pathogen Trichomonas vaginalis. J Microbiol Immunol infect 2012. Published Online First: 21 Aug 2012.10.1016/j.jmii.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Conrad MD, Gorman AW, Schillinger JA, et al. Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis 2012;6:e1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honigberg BM. Host cell-Trichomonad interactions and virulence assays in in vitro systems. In: Honigberg BM, ed. Trichomonads parasitic in humans. New York: Springer-Verlag, 1990:155–212 [Google Scholar]
- 22.de Miguel N, Lustig G, Twu O, et al. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics 2010;9:1554–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Miguel N, Riestra A, Johnson PJ. Reversible association of tetraspanin with Trichomonas vaginalis flagella upon adherence to host cells. Cell Microbiol 2012;14:1797–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessi D, Rappelli P, Diaz N, et al. Mycoplasma hominis and Trichomonas vaginalis: a unique case of symbiotic relationship between two obligate human parasites. Front Biosci 2006;11:2028–34 [DOI] [PubMed] [Google Scholar]
- 25.Morada M, Manzur M, Lam B, et al. Arginine metabolism in Trichomonas vaginalis infected with Mycoplasma hominis. Microbiology 2010;156(Pt 12 Special issue on Trichomonas vaginalis):3734–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsmark UC, Foster PG, Sicheritz-Ponten T, et al. Patterns of prokaryotic lateral gene transfers affecting parasitic microbial eukaryotes. Genome Biol 2013;14:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 2012;10:323–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rughooputh S, Greenwell P. Trichomonas vaginalis: paradigm of a successful sexually transmitted organism. Br J Biomed Sci 2005;62:193–200 [DOI] [PubMed] [Google Scholar]
- 29.Goodman RP, Ghabrial SA, Fichorova RN, et al. Trichomonasvirus: a new genus of protozoan viruses in the family Totiviridae. Arch Virol 2011;156: 171–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fichorova RN, Lee Y, Yamamoto HS, et al. Endobiont viruses sensed by the human host—beyond conventional antiparasitic therapy. PLoS One 2012;7:e48418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. doi: 10.1136/sextrans-2012-051006. Fiori PL, Diaz N, Cocco AR, et al. Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex Transm Infect 2013;89: 449–54. [DOI] [PubMed] [Google Scholar]