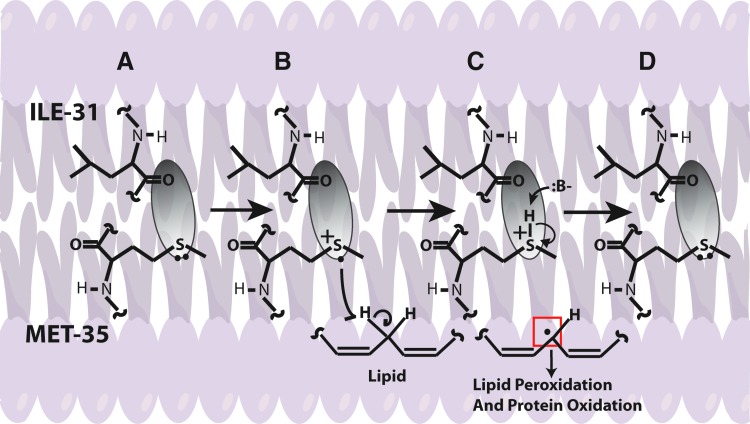
FIG. 7.
A proposed mechanism for the Aβ-induced free radical stress hypothesis. As shown, the electron density surrounding the sulfur atom of Met-35 is pulled away by the more electronegative oxygen of the carbonyl located on the peptide backbone at the position of Ile-31. As discussed, the carbonyl is within Van der Waals distance to the sulfur, which primes the lone pair on the sulfur for one-electron oxidation, forming the sulfuranyl radical. Because this occurs within the bilayer, unsaturated lipids are present, allowing for an allylic hydrogen atom abstraction by the sulfuranyl radical to eventually form a reduced Met-35 that recycles back upon deprotonation to the starting conditions for another cycle. The carbon centered radical may then go on to undergo peroxidation to create reactive aldehydes or may directly interact with another protein or lipid in a radical propagation step. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars