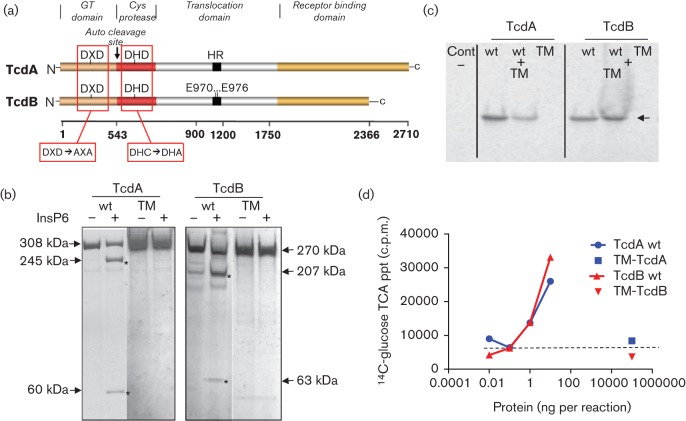
Fig. 3.
Structural features and GT activities of TM toxins. (a) Schematic highlighting mutations targeting GT catalytic and auto-proteolytic processing activities, modified from illustration in Jank et al. (2007a). Also indicated is the position of pore-inducing glutamate residues E970 and E976 within the TcdB hydrophobic region (HR). (b) InsP6-induced auto-proteolytic cleavage of purified wt TcdA/B and TM-TcdA/B toxins. Purified proteins (~1 µg) were incubated in the presence or absence of InsP6 at RT overnight. The cleavage products were separated by SDS-PAGE and stained with silver. Asterisks mark the molecular sizes of proteolytic cleavage products. (c) GT activity of native toxins and TM toxins. wt TcdA/B (1 ng) or TM-TcdA/B (100 µg) were incubated with RhoA GTPase in the presence of UDP-14C-glucose for 2 h at 30 °C. The activity of 100 µg TM-TcdA/B in the presence of 1 ng wt TcdA/B (wt+TM) was also assessed as control. The reaction mixture was resolved on SDS-PAGE and radioactive bands were visualized by PhosphorImager. Arrow indicates the 28 kDa 14C-labelled Rho GTPase protein band. (d) Concentration dependent toxin GT activity (at >0.1 ng ml−1) and lack of detectable activity (at 100 µg) for TM-antigens. After 2 h at 30 °C, reaction products were TCA precipitated and counted. Dotted line indicates the level of background 14C-glucose counts recovered from control reactions lacking TcdA or TcdB protein.