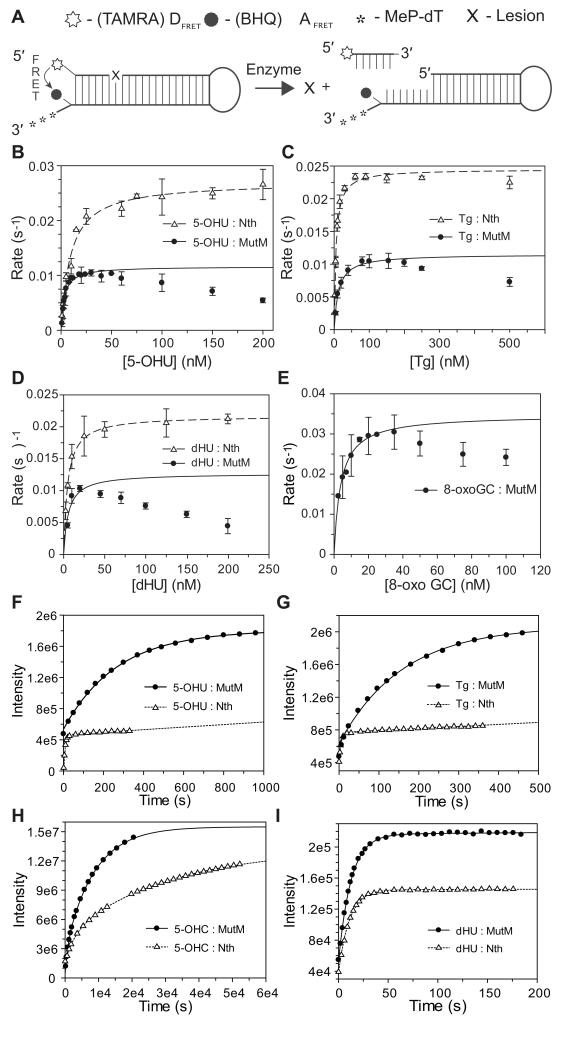
Figure 2.
Catalytic properties of Nth and MutM.
(A) Substrates used for assays of bi-functional glycosylases. A damaged base (X) is removed by glycosylase activity, while lyase activity cleaves the DNA strand. After cleavage, the six base strand of DNA with a 5′ fluorescent TAMRA FRET donor (DFRET) is no longer in close proximity to the BHQ2 FRET acceptor (AFRET), allowing fluorescence and indicating product release. (B, C, D, and E) Kinetics of MutM (closed circles) and Nth (open triangles) with various DNA lesions. Data where plotted as initial rates against the concentration of substrate and fitted to the Michaelis-Menten’s equation. Data points that are lowered by substrate-product inhibition were omitted from the fit as these provide low and inaccurate estimates of rates. Error bars show the S.D. of at least three measurements. Determination of the reaction rates under steady state conditions (i.e. at least 10-fold excess of substrate to enzyme) for MutM was problematic due to product inhibition; 8-oxoG·C is a substrate for MutM but not Nth (not shown). (F, G, H and I) Single-turnover kinetics of Nth and MutM. Fluorescent hairpin substrates containing the lesions indicated were mixed with 1 μM of enzyme, and reactions followed using a FluoroMax®-3 fluorimeter. Data were fitted using Grafit® 6. Key rate constants are displayed in Table 1. Different saturation levels of Nth and MutM are caused by quenching of fluorescence by binding of Nth. Panels F and G show that reaction rates are faster with Nth (open triangles) than MutM (closed circles) with 5-OHU and Tg under-single turnover conditions (performed with an at least 10-fold excess of enzyme to substrate). (H and I) Nth and MutM display similar rate constants with dHU, and limited activity against 5-OHU but only over several hours.