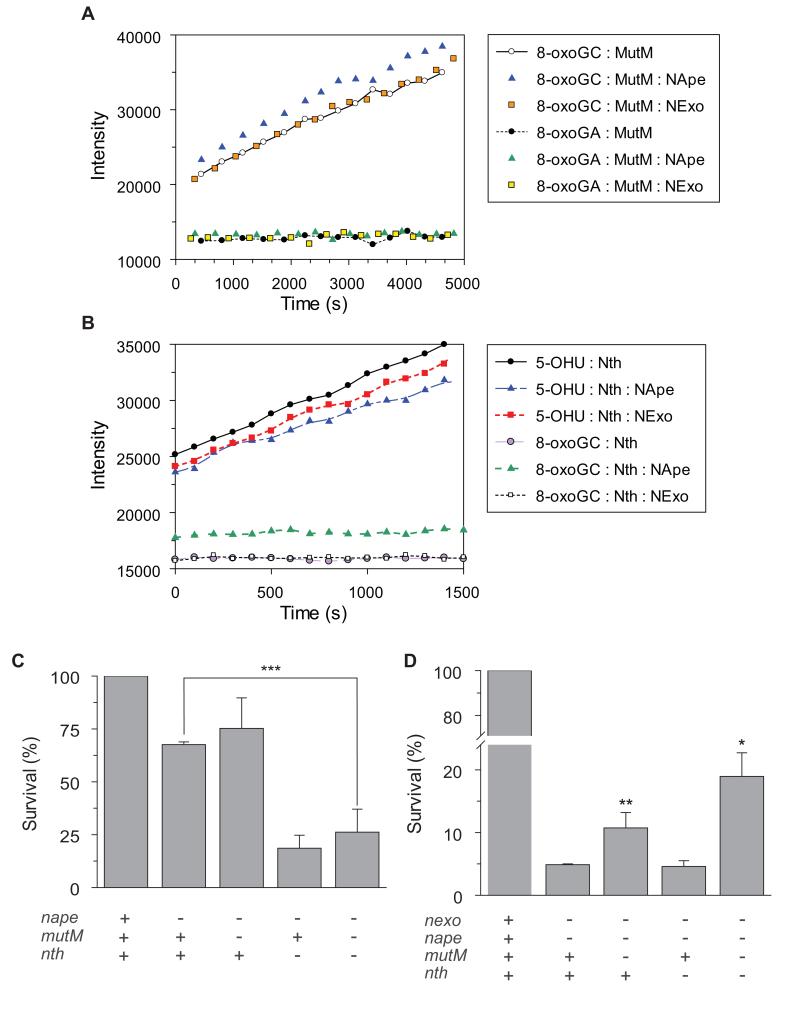
Figure 3.
Lack of co-operation between glycosylase and 3′ processing enzymes.
(A and B) Steady-state glycosylase assays were performed in the presence or absence of NExo or NApe. Hairpin substrates (100 nM) were mixed with 10 nM NExo, NApe or reaction buffer alone. After 5 mins, MutM (A) or Nth (B) was added to reactions at a final concentration of 1 nM. Time points are shown after the addition of the glycosylase. Genetic analysis of interactions between bi-functional glycosylases and 3′ processing enzymes. (C) NApe and Nth share redundant functions in the meningococcus; removal of Nth but not MutM impairs the survival of a nape mutant in the presence of oxidative stress while loss of MutM or both bi-functional glycosylases improves the survival of cells lacking both 3′ processing enzymes (D). Bacteria were incubated with H2O2 (0.4 mM for C and 0.1 mM for D) or PBS for 30 min and then recovered by plating to solid media. The percentage killing was calculated by comparing the number of CFU in the presence and the absence of the oxidizing agent. + and − indicate the presence and absence of enzymes, respectively. Results show the percentage survival of strains relative to the nexo and nape mutants. Error bars show the SD of assays performed in triplicate on three occasions (***, **, and *indicate p < 0.001, 0.003, and 0.04, respectively, 1-column Student’s t test).