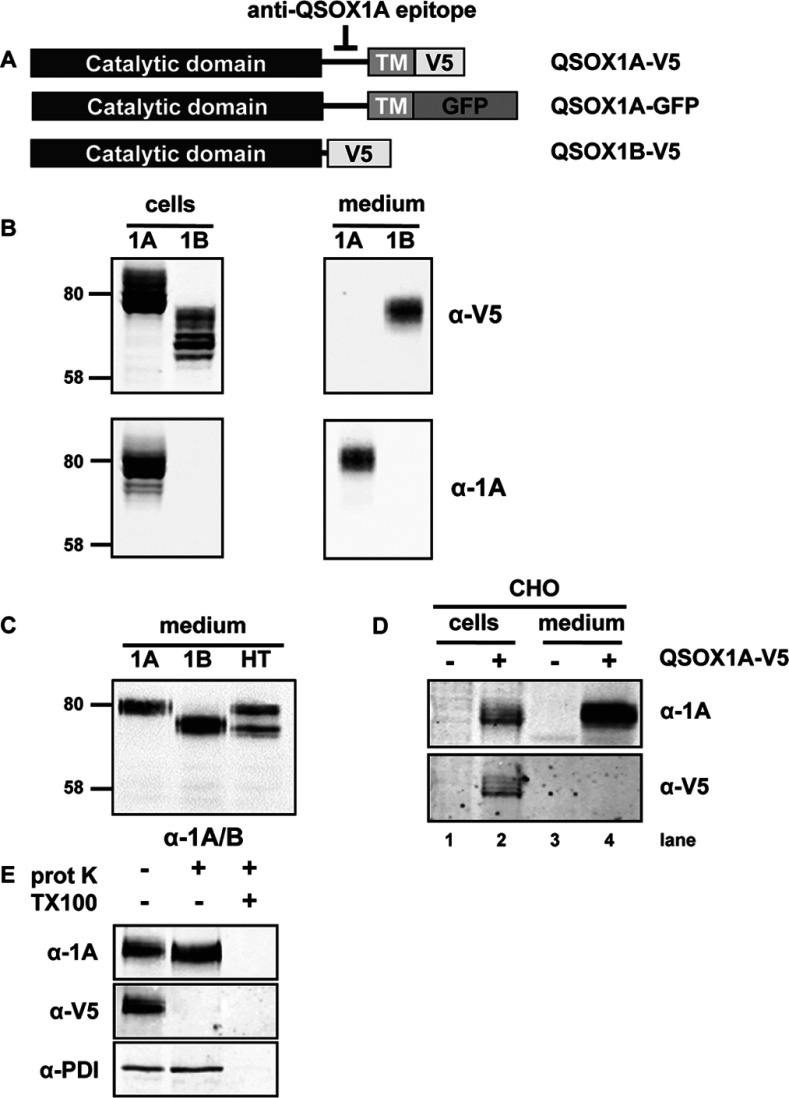
Figure 1. Expression and secretion of human QSOX1A.
(A) The QSOX1 constructs used in the present study are shown. Upper panel: the QSOX1A sequence including the C-terminal single-pass TM domain followed by a V5-tag (V5). The epitope detected by the anti-QSOX1A antibody is located on a linker that connects the Erv and TM domain. Middle panel: the same as above, only the C-terminal V5 tag is changed to a GFP tag. Lower panel: V5-tagged QSOX1B. (B) Western blots showing the expression and secretion of QSOX1A–V5 (1A) and QSOX1B–V5 (1B). Cell lysates were prepared from HT1080 cells stably overexpressing V5-tagged forms of either QSOX1A or QSOX1B. The secretion of proteins was allowed for 3 h into serum-free medium followed by TCA precipitation. Nitrocellulose membranes were co-probed for V5 (secondary DyLight680) and QSOX1A (secondary DyLight800). Molecular masses of marker proteins are indicated (in kDa). (C) Secretion of overexpressed forms of QSOX1A–V5 (1A), QSOX1B–V5 (1B) and endogenous QSOX1 forms (HT) is shown. Secretion of QSOX1 into serum-free medium was allowed for 3 h and proteins were affinity-purified using concanavalin A–Sepharose. Gel loading was adjusted according to the estimated secretion levels of QSOX1 from different cell lines. Nitrocellulose membranes were probed using an antibody detecting both forms of QSOX1 (α-1A/B). Molecular masses of marker proteins are indicated (in kDa). (D) A CHO cell line stably expressing human QSOX1A–V5 (+) was compared to untransfected CHO cells (−). Cell lysates and medium were prepared and analysed as described in (B). (E) Proteinase K protection assay. Semi-permeabilized QSOX1A–V5-expressing cells were incubated in the presence (+) or absence (−) of proteinase K (prot K) and/or Triton X-100 (TX100). Nitrocellulose membranes were co-probed for the V5 and QSOX1A epitopes (see B) and PDI (α-PDI), an ER marker indicating integrity of the inner membranes.