Abstract
Objective:
The purpose of this review is to assess whether evidence supports a favorable risk/benefit profile for pediatric antidepressant use and reconsideration of the black box.
Method:
The review examines studies post-black box purporting to show declines in pediatric antidepressant use and rising youth suicide, summarizes evidence for efficacy and safety of pediatric antidepressants, and discusses irregularities in recent meta-analyses of fluoxetine for youth.
Results:
Pediatric antidepressant prescription did not significantly decline post-black box and youth suicide has risen only in recent years. Recent meta-analyses fail to undermine evidence that antidepressants are associated with increased risk of suicidality in youth.
Conclusions:
First line prescription of antidepressants for youth is not advisable. The black box and international warnings on pediatric use of antidepressants are warranted. Wider availability of psychosocial options for depressed youth is recommended.
Keywords: antidepressants, SSRIs, children, adolescents, suicide, black box
Résumé
Objectif:
Cette revue a pour but d’évaluer si les données probantes soutiennent un profil risques-avantages favorable à l’utilisation d’antidépresseurs pédiatriques, et de réexaminer la boîte noire.
Méthode:
La revue examine les études postérieures à la boîte noire censées démontrer l’utilisation décroissante d’antidépresseurs pédiatriques et la montée du suicide chez les adolescents, résume les données probantes sur l’efficacité et l’innocuité des antidépresseurs pédiatriques, et présente les irrégularités des récentes méta-analyses de la fluoxétine pour adolescents.
Résultats:
La prescription d’antidépresseurs pédiatriques n’a pas connu de baisse significative postérieurement à la boîte noire, et le suicide chez les adolescents n’a augmenté que dans les dernières années. Les méta-analyses récentes n’infirment pas les données probantes de l’association des antidépresseurs à un risque accru de suicidabilité chez les adolescents.
Conclusions:
La prescription en première intention d’antidépresseurs à des adolescents n’est pas à conseiller. La boîte noire et les mises en garde internationales sur l’utilisation pédiatrique d’antidépresseurs sont justifiées. Une offre plus variée d’options psychosociales pour les adolescents déprimés est recommandée.
Keywords: antidépresseurs, ISRS, enfants, adolescents, suicide, boîte noire
With the rapid acceleration of selective serotonin reuptake inhibitor (SSRI) prescription to youth from 1988 to 2004 (Delate, Gelenberg, Simons, & Motheral, 2004; Zito, 2002), safety became a pressing issue. Over time, a consistent finding of increased risk of suicidality for children and adolescents taking SSRIs emerged (Hammad, 2004; Whittington et al., 2004). In response, the US Food and Drug Administration (FDA) issued a series of advisories culminating in a black box warning for all antidepressants for those under age 18 (FDA, 2004, October 15). A stronger reaction ensued in the UK. The Medicines and Healthcare Products Regulatory Authority (http://www.mhra.gov.uk/home/groups/pl-p/documents/drugsafetymes-sage/con019472.pdfS) banned all antidepressants for those under 18 with the exception of fluoxetine, which could only be used for those over age eight when psychosocial intervention failed. Similarly, Health Canada advised SSRI-treated patients under 18 to consult physicians regarding benefit versus risk and cautioned that SSRIs were not approved for pediatric use (http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2004/13053a-eng.php).
Two recent studies challenged these warnings. Gibbons, Brown, Hur, Davis, & Mann, (2012) re-analyzed four trials and reported no significant suicidality for those under age 18. The authors contended that this contradicted findings that were the basis of the black box. A companion article found “impressive effects on clinically interpretable outcomes” for fluoxetine in the same four trials (Gibbons et al., 2012), concluding that the risk/benefit equation that led to the black box should be revisited. Following these two publications, media sources announced the safety of not only fluoxetine but all SSRIs for youth (e.g., Lowry, 2012; Roan, 2012).
Nearly a decade after the black box, controversy surrounding the safety and efficacy of pediatric SSRI use remains. This article evaluates claims that the black box and similar international warnings have not served the interests of children and adolescents and ought to be reconsidered. Specifically, it examines the proposed connection between prescription trends and youth suicide and whether new evidence supports a favorable risk/benefit profile.
Impact of FDA Regulations on Pediatric Antidepressant Use and Suicidality
Controversy surrounding the warnings about antidepressants and youth peaked in 2007 when some pointed to increased rates of youth suicide and speculated that, because of the FDA’s actions, lower prescription rates left many untreated (e.g., Gibbons et al., 2007; Libby et al., 2007). However, significant reductions of pediatric SSRI prescriptions are not substantiated. The steep rise in prescriptions of antidepressants, most notably SSRIs, for US youth in the five years prior to 2004 is well documented (Chen & Toh, 2011; Olfson, Marcus, & Druss, 2008; Zito, 2002). As might be expected, studies evaluating the impact of FDA warnings (Libby et al., 2007; Nemeroff et al., 2007; Olfson et al., 2008; Pamer et al., 2010) found marked differences between forecasted and actual rates following the black box advisory. These investigations did not find a significant decrease in prescription rates but rather a reduction in prescription growth.
Regarding actual rates, Gibbons et al. (2007) used a randomly selected sample from IMS pharmacy data and reported that pediatric prescriptions, expressed as a percentage of 2003 rates, declined approximately 10% for ages 15 to 19, 14% for ages 11–14, and 20%, ages ten and under from 2003 to 2005. Since this study only presents rates graphically without tabulated data, statistical comparison is impossible. In contrast, all other investigations examining actual rates of prescriptions found a pattern of stable, not declining, rates. According to Libby et al. (2007), there were no statistically significant changes in mean levels of prescriptions in their pre- and post-advisory analysis. In addition, Olfson et al. (2008) found a non-significant decline in the rate of use of antidepressants for ages 6–17 for the black box study period. In fact, SSRI use, with the exception of paroxetine, increased through 2004, and new use of antidepressants in the year following the black box did not significantly decrease. Similarly, Pamer et al. (2010) reported that observed mean use rates post-February 2004 through 2006 did not significantly differ from the 2002 through February 2004 period. Finally, Chen and Toh (2011) examined prescribing data from ambulatory settings in the US from 1998 through 2007 and reported that antidepressant prescriptions for children with major depressive disorder rose post-advisories. In sum, reports of significantly reduced antidepressant prescription following the black box are not empirically supported.
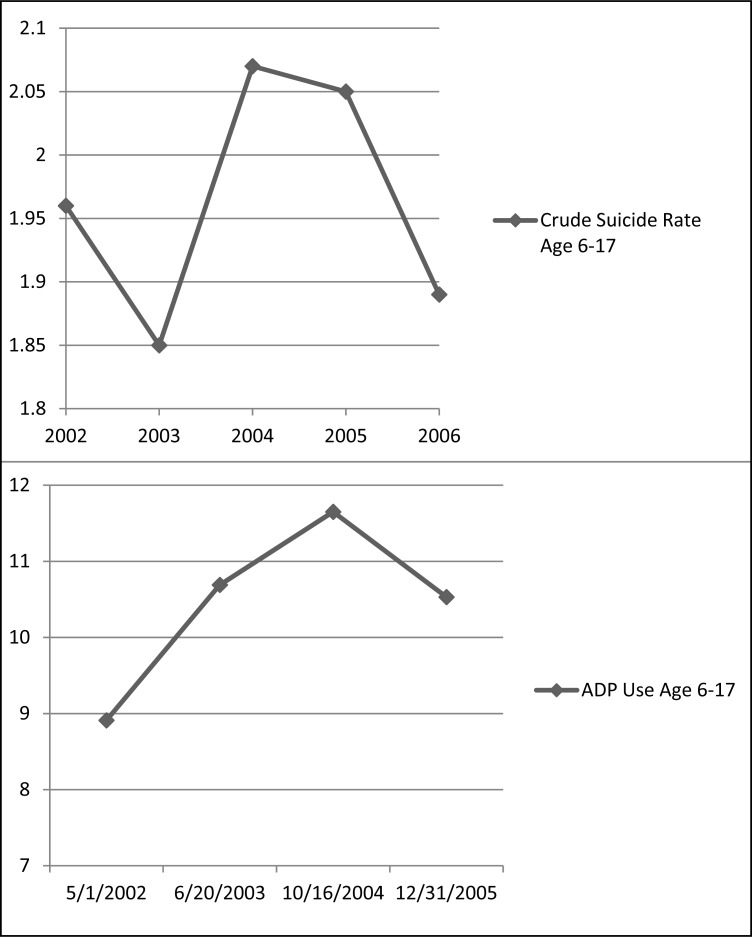
The second premise of the proposed link between declining prescriptions and youth suicide involves suicide rates after the black box. One well-publicized study graphically depicted rising youth suicides in 2003–2004 prior to hypothesized declining prescription rates (Gibbons et al., 2007), even though the investigation posited a link between youth suicide and declining prescriptions. Moreover, the 2003–2004 Centers for Disease Control (CDC) suicide data contradict the assertion that the black box increased youth suicide. According to the CDC, rates of suicide for the age range 6–17 rose slightly from 2003–2004 and then declined through 2007. As noted, examining the time frame through 2004, Olfson et al. (2008) found rising prescription rates for youth ages 6–17 for the pre-warning observation period; for the black box period, there was a non-significant decline in the rate of use of any antidepressant. Connecting these data with the CDC data, youth suicides rose in concert with rising prescription rates and declined when rates stabilized (see Figure 1). Claims, then, that decreasing pediatric anti-depressant utilization corresponded with increasing youth suicide are factually inaccurate.
Figure 1.
Top: Suicide rates (per 100,000) for ages 6–17 (2002–2006) (Centers for Disease Control and Prevention, www.cdc.gov/ncipc/wisqars). Bottom: Antidepressant (ADP) use per 1000 across pre-warning period (May 1, 2002 – October 16, 2004) to black box period (October 16, 2004 to December 31, 2005)
Adapted from Olfson et al., 2008). Last data point calculated from Olfson et al. (2008) reported non-significant 9.6% reduction from October 16, 2004 to December 31, 2005.
From 2007 to 2010, the last available data year, youth suicide (age 6–17) increased from 1.68 per 100,000 to 2.02.This trend can be analyzed alongside more recent prescription reports. From 2007–2008, antidepressants were the third most frequently used drug by adolescents aged 12–19 (Gu, Dillon, & Burt, 2010).1 The year 2009–2010 saw an increase in pediatric antidepressant prescription to the highest rates since 2004 (Chai et al., 2012). These data indicate that the recent uptick in youth suicide has occurred as prescriptions approached 2003 levels, again disconfirming claims that less antidepressant prescribing accompanied increases in youth suicide.
Risk and Benefit Revisited
After 2007, debates regarding the potential harm of decreased juvenile antidepressant prescription receded. However, two recent meta-analyses (see, Gibbons, Brown et al., 2012; Gibbons, Hur et al., 2012) have re-awakened the controversy. A recent review of these two studies and an older meta-analysis (see Bridge et al., 2007) concluded that antidepressants “may not increase suicidality in pediatric patients and may be effective in treating juvenile depression” (Adegbite-Adeniyik, Gron, Rowles, Demeter, & Findling, 2012).
In light of the potential impact of these recent studies and their media depiction on prescription practices, it is important to evaluate their contribution to the extensive evidence regarding pediatric antidepressant safety and efficacy. Regarding safety, the FDA’s pooled analysis of 4400 youth revealed an average risk of suicidality of 4% in medication treated patients, twice the 2% placebo risk (Hammad, Laughren, & Racoosin, 2006), giving rise to the black box warning (FDA, 2004, October, 15). Around this time, Whittington et al. (2004) reviewed published and unpublished studies and found that SSRIs entailed an increased risk of serious adverse events, most notably suicidal thinking and behaviour. An additional FDA analysis of 22 short-term randomized controlled trials (RCT) of pediatric antidepressants reported a rate of serious suicidal events for drug treatment almost twice that of placebo (Mosholder & Willy, 2006). Additionally, the FDA meta-analyzed 372 RCTs and reported that the link between suicide-related events and antidepressants was age dependent; persons under 25 were at the greatest risk, and this risk increased as age decreased (Laughren, 2006). In support of these findings, a case control study examining two cohorts of children and adolescents age 6–18 with matched illness severity following inpatient treatment for depression found that the anti-depressant-treated cohort was significantly more likely to attempt and complete suicide (Olfson, Marcus, & Shaffer, 2006).
Testing three conditions, SSRI treatment, cognitive behavioural therapy (CBT) plus suicide prevention, or a combination, the Treatment of Adolescent Suicide Attempters (TASA; Brent et al., 2009; Vitiello, Brent, et al., 2009) study examined suicidal events, risk factors, and the course of depression for 124 adolescents with at least moderate depressive symptoms who had previous suicide attempts. After six months of treatment, 22% taking the SSRI had a suicide event compared with 6.7% not taking the drug.
More recently, a review of the emergence of activation in depressed and anxious youth treated with antidepressants found overall excessive arousal rates of treated youth 3–10 times higher than placebo (Offidan, Fava, Tomba, & Baldessarini, 2013). Given the potential link between activation and suicidality, these findings are cause for concern.
Faced with substantial evidence of heightened risks, researchers have explored how much risk is tolerable given the medication’s benefits. To this end, the FDA independently examined both published and unpublished trials and found that the investigated antidepressant was more effective than placebo on primary measures in only three out of 15 trials (Hammad et al., 2006). In a study of 13 trials, Bridge et al. (2007) reported pooled absolute rates of responses for youth treated with antidepressants of 60% compared with 50% for placebo. Using a similar database, another meta-analysis also reported an approximate 10% difference in response rates (Tsapakis, Soldani, Tondo, & Baldessarini, 2008). This study’s authors concluded that antidepressants have little clinical efficacy for depressed juveniles. Notably, the small effects found in pediatric antidepressant trials have been found on clinician-rated measures only, not patient or parent-rated measures (Jureidini et al., 2004).
In many cases, even clinician-rated measures show no efficacy over placebo. For example, in the two RCTs that garnered FDA approval for fluoxetine for youth ages 8–17, only two of four clinician-rated measures (Emslie et al., 1997) and three of six (Emslie et al., 2002) indicated a difference favoring fluoxetine. Both studies failed to find a statistical difference between fluoxetine and placebo on primary endpoint measures (Jureidini et al., 2004). An FDA statistical review of these trials concluded: “...the sponsor did not win on these two pediatric depression studies...” The evidence for efficacy, based on the pre-specified endpoint, is not convincing” (FDA, 2000, October 10).
The NIMH-funded Treatment of Adolescent Depression Study (TADS; March et al., 2004; March et al., 2007), a 36-week trial evaluating fluoxetine for youth (ages 12–17) is often cited as evidence that combining fluoxetine and psychotherapy is more effective than medication or psychotherapy alone and is protective of suicidality (e.g., see (http://www.nimh.nih.gov/healthinformation/tads.cfm). In the 12-week RCT stage, TADS compared fluoxetine alone, CBT alone, CBT plus fluoxetine, and placebo. End-point comparisons favored the combined arm. However, the combined treatment was not blinded and, unlike the other groups, received all intervention components (medication, psychotherapy, psychoeducation, family therapy, and supportive pharmacotherapy monitoring), creating a significant disparity in treatment dose favoring this arm. Without a combined CBT/placebo group, the relative contributions of the fluoxetine and CBT to overall efficacy cannot be determined. The FDA did not count TADS as a positive study for the SSRI given the failure of fluoxetine to separate from placebo on one of its two primary outcome measures. After the TADS acute 12-week stage, partial and non-responders to all treatments were openly treated (March et al., 2007). All treatment conditions converged by 30 weeks and remained so by week 36.
Under simulated real-world conditions, TORDIA (Treatment of Resistant Depression in Adolescents; Brent et al., 2008; Emslie et al., 2010) compared outcomes for 334 adolescents who initially had not responded to medication and were randomized to switch to a different one (fluoxetine, citalopram, or venlafaxine) or a different medication plus CBT. At week 24, there were no significant differences between treatments for rates and time to remission and relapse. In the first 12 weeks, there were 68 (20%) harm-related events including 18 suicide attempts. In the second 12 weeks, 11% of the adolescents experienced a harm-related event and 7%, a non-suicidal self-injury. If non-suicidal self-injuries were counted as harm-related, as in the first 12-weeks, 18% of trial participants, all taking either an SSRI or venlafaxine, were involved in self-harming behaviour.
In sum, research has consistently pointed toward an unfavorable antidepressant risk/benefit profile for the pediatric population. With this as a backdrop, Gibbons, Brown, et al. (2012) and Gibbons, Hur, et al. (2012) re-examined fluoxetine safety and efficacy for youth. Their meta-analyses of three Eli Lilly funded trials and the TADS found no increased risk of suicide behaviour or thinking related to fluoxetine for juvenile subjects as well as significant benefit. A closer look, however, reveals noteworthy irregularities. One of the four trials (Kratochvil et al., 2005, identified as LYAQ, the industry identification) is not a fluoxetine trial but rather a combined atomoxetine and fluoxetine trial for youth diagnosed with ADHD with concurrent symptoms of depression or anxiety. Moreover, the baseline mean score for depression in LYAQ was 44.2, the lower end of a moderately depressed range on the Children’s Depression Rating Scale-Revised (CDRS-R; Poznanski & Mokros, 1996). In contrast, the other three trials had a mean baseline score of 58.1, close to a score of severe depression (61). The LYAQ, therefore, is inappropriate for inclusion in both meta-analyses, thereby invalidating the aggregated data, results, and conclusions.
Gibbons, Brown, et al. (2012) did not examine suicidal ideation adverse event reports, even though these are considered a suicide event by the Columbia Classification Algorithm of Suicide Assessment (Posner, Oquendo, Gould, Stanley, & Davies, 2007), FDA analyses (Hammad et al., 2006; Mosholder & Willy, 2006), and the TADS. Instead, this study used one item of a clinician-rated scale, the CDRS-R, for its primary suicide analysis. Spielmans, Jureidini, Healy, and Purssey (2013) point out that the CDRSR was designed to measure efficacy, not suicidality, and noted that top FDA officials, Thomas Laughren and Robert Temple, concurred that a depression rating scale does not provide a valid signal for suicidality. Gibbons, Brown, et al.’s (2012) inconsistency with standard pediatric suicidality assessment most likely resulted in under-representation of suicide-related events.
Additionally, Gibbons, Brown, et al. (2012) contradicts safety findings from the TADS, one of its primary sources of data. Examining suicide events according to whether subjects were on fluoxetine at the time of the event indicates 36 events for fluoxetine-treated compared with eight for non-fluoxetine (17 on fluoxetine attempted suicide, one not on fluoxetine; Vitiello, Silva, et al., 2009).2,3 Beyond 12 weeks, 15 fluoxetine-takers had a suicidal event compared with none for those not taking the SSRI. These data starkly contrast with the conclusions in Gibbons, Brown, et al. (2012).
Re-analyzing the benefits of fluoxetine for youth, Gibbons, Hur, et al. (2012) reported a 4.62 point difference in the estimated average rate of change between fluoxetine and placebo on the CDRS-R. The authors translated this difference to an estimated fluoxetine combined response/remission rate of 54.1% over placebo, significantly at odds with the reported results of the three fluoxetine trials included in the analysis. In conclusion, recent investigations of safety and efficacy of fluoxetine for youth contain significant confounds that discredit their findings.
Conclusions
Despite no convincing evidence to supplant a decade of research indicating limited efficacy and potential harm for youth taking antidepressants, the black box and similar warnings continue to be contested. When Gibbons, Brown, et al. (2012) and Gibbons, Hur, et al. (2012) were first published, press reports distorted their findings. For example, the National Public Radio’s health blog proclaimed: “A Fresh Look at Antidepressants Finds Low Risk of Suicide” (Spiegel, 2012). The LA Times reported “...analysis suggests there is no reason to believe that antidepressants influence suicidal thinking in kids” (Roan, 2012), and the popular online site, Medscape, heralded “No Link Between Antidepressants and Suicide in Kids” (Lowry, 2012). Such headlines, reaching so many in a sound-bite culture, could reopen the door for unrestrained prescribing, precisely what the black box was intended to prevent. Greater publicity regarding empirical support for the black box and limitations of recent investigations are required to counter misleading and potentially harmful narratives.
The extant literature reviewed herein convincingly suggests that first-line prescription of antidepressants for the pediatric population is not advisable. There is justification, however, for supportive monitoring (Cheung et al., 2007) and watchful waiting without formal intervention in less serious presentations (Jureidini, 2009). Funding for research, training, and implementation of non-medication options for pediatric depression deserves greater priority. Until evidence emerges beyond what is currently available, reconsideration of the black box needs to proceed with great caution and conscientious review. For now, pediatric antidepressant prescription belongs “inside the box.”
Acknowledgements/Conflicts of Interest
The authors have no financial relationships to disclose.
Footnotes
Between 2005 and 2009, pediatric SSRI prescription rates significantly increased in the UK by nearly 11% (Wijlaars, Nazareth, & Petersen, 2012) following a decline after the Committee on Safety of Medicine’s 2003 advisory against SSRIs other than fluoxetine to pediatric patients.
We were first alerted to this data by Robert Whitaker’s blog (http://www.madinamerica.com/2012/02/the-real-suicide-data-from-the-tads-study-comes-to-light/). Whitaker credits Göran Högberg from Stockholm, Sweden for directing him to this previously unpublished data.
Vitiello, Silva et al. (2009) state that the eleven subjects were placed on an SSRI, without specifying that the SSRI was fluoxetine. However, given that these subjects remained in the TADS trial, it is assumed that the SSRI was fluoxetine.
References
- Adegbite-Adeniyi C, Gron B, Rowles BM, Demeter CA, Findling RL. An update on antidepressant use and suicidality in pediatric depression. Expert Opinion Pharmacotherapy. 2012;13(15):2119–2130. doi: 10.1517/14656566.2012.726613. [DOI] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Zelazny J. Switching to another SSRI or to venlafaxine with or without cognitive behavioural therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Greenhill M, Comptom S, Emslie G, Wells K, Walkup J, Turner B. The treatment of adolescent suicide attempters study (TASA): Predictors of suicidal events in an open treatment trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:987–996. doi: 10.1097/CHI.0b013e3181b5dbe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, lyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):22–32. doi: 10.1542/peds.2011-2879. [DOI] [PubMed] [Google Scholar]
- Chen S-Y, Toh S. National trends in prescribing antidepressants before and after an FDA advisory on suicidality risk in youth. Psychiatric Services. 2011;62:727–733. doi: 10.1176/ps.62.7.pss6207_0727. [DOI] [PubMed] [Google Scholar]
- Cheung AH, Zuckerbrot RA, Jensen PS, Ghalib K, Laraque D, Stein REK. Guidelines for adolescent depression in primary care (GLAD-PC): II. Treatment and ongoing management. Pediatrics. 2007;120:e1313. doi: 10.1542/peds.2006-1395. [DOI] [PubMed] [Google Scholar]
- Delate T, Gelenberg AJ, Simmons VA, Motheral BR. Trends in the use of antidepressants in a national sample of commercially insured pediatric patients, 1998 to 2002. Psychiatric Services. 2004;55(4):387–391. doi: 10.1176/appi.ps.55.4.387. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Jacobson JG. Fluoxetine for acute treatment of depression in children and adolescents: A placebo-controlled, randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KG, Brent D. Treatment resistant depression in adolescents (TORDIA): Week 24 outcomes. American Journal of Psychiatry. 2010;167:782–791. doi: 10.1176/appi.ajp.2010.09040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelman J. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Archives of General Psychiatry. 1997;54(11):1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- FDA Statistical review. 2000. Oct 10, Retrieved from: www.antidepressantsfacts.com/doctor-Emslie-3.pdf.
- FDA FDA Public Health Advisory: Suicidality in children and adolescents being treated with antidepressant medications. 2004. Oct 15, Retrieved from: http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm161679.htm.
- Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Erkens JA, Mann JJ. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. American Journal of Psychiatry. 2007;164:1356–1363. doi: 10.1176/appi.ajp.2007.07030454. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hur K, Brown CH, Davis JM, Mann J. Benefits from antidepressants: Synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Archives of General Psychiatry. 2012;69(6):572–579. doi: 10.1001/archgenpsychiatry.2011.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Brown CH, Hur K, Davis JM, Mann J. Suicidal thoughts and behaviour with antidepressant treatment: Reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Archives of General Psychiatry. 2012;69(6):580–587. doi: 10.1001/archgenpsychiatry.2011.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief. 2010 2010 Sep;42 Retrieved from http://www.cdc.gov/nchs/data/databriefs/db42.htm. [PubMed] [Google Scholar]
- Hammad T. Relationship between psychotropic drugs and pediatric suicidality. 2004. pp. 1–131. [ http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf]. FDA Accessed June 2005.
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Archives of General Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- Jureidini JN, Doecke CJ, Mansfield PR, Haby MM, Menkes DB, Tonkin AI. Efficacy and safety of antidepressants for children and adolescents. British Medical Journal. 2004;328:879–883. doi: 10.1136/bmj.328.7444.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jureidini J. How do we safely treat depression in children, adolescents, and young adults? Drug Safety. 2009;32(4):275–282. doi: 10.2165/00002018-200932040-00002. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ, Newcorn JH, Arnold E, Duesenberg D, Emslie GJ, Quintana H, Biederman J. Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(9):915–924. doi: 10.1097/01.chi.0000169012.81536.38. [DOI] [PubMed] [Google Scholar]
- Laughren T. Memorandum: Overview for the December 13 meeting of Psychopharmacologic Drugs Advisory Committee. Food and Drug Administration; 2006. Dec 13, 2006. Retrieved from: www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-fda.pdf. [Google Scholar]
- Libby AM, Brent DA, Morrato EH, Orton HD, Allen R, Valuck RJ. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. The American Journal of Psychiatry. 2007;164(6):884–891. doi: 10.1176/ajp.2007.164.6.884. [DOI] [PubMed] [Google Scholar]
- Lowry F. No link between antidepressants and suicide in kids: Study calls FDA black box warning into question. 2012. Feb 21, Retrieved from: http://www.medscape.com/viewarticle/758917?src=emailthis.
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Severe J. Fluoxetine, cognitive-behavioural therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- March JS, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Severe J. The Treatment for Adolescents With Depression Study (TADS): Long-term effectiveness and safety outcomes. Archives of General Psychiatry. 2007;64:1132–1143. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- Mosholder AD, Willy M. Suicidal adverse events in pediatric randomized, controlled clinical trials of antidepressant drugs are associated with active treatment: A meta-analysis. Journal of Child & Adolescent Psychopharmacology. 2006;16(1–2):25–32. doi: 10.1089/cap.2006.16.25. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Kalali A, Keller MB, Charney DS, Lenderts SE, Cascade EF, Schatzberg AF. Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Archives of General Psychiatry. 2007;64(4):466–472. doi: 10.1001/archpsyc.64.4.466. [DOI] [PubMed] [Google Scholar]
- Offidan E, Fava GA, Tomba E, Baldessarini RJ. Excessive mood elevation and behavioural activation with antidepressant treatment of juvenile depressive and anxiety disorders: A systematic review. Psychotherapy and Psychosomatics. 2013;82(3):132–141. doi: 10.1159/000345316. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Druss BG. Effects of Food and Drug Administration warnings on antidepressant use in a national sample. Archives of General Psychiatry. 2008;65(1):94–101. doi: 10.1001/archgenpsychiatry.2007.5. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults. Archives of General Psychiatry. 2006;63:865–872. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- Pamer CA, Hammad TA, Wu YT, Kaplan S, Rochester G, Governale L, Mosholder AD. Changes in US antidepressant and antipsychotic prescription patterns during a period of FDA actions. Pharmacoepidemiology and Drug Safety. 2010;19(2):158–174. doi: 10.1002/pds.1886. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. American Journal of Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Mokros H. Children’s Depression Rating Scale – Revised (CDRS-R) Los Angeles, CA: WPS; 1996. [Google Scholar]
- Roan S. Study questions antidepressant link to suicide in kids. 2012. Feb 6, Retrieved from: http://articles.latimes.com/2012/feb/06/news/la-heb-antidepressants-kids-20120206.
- Spiegel A. A fresh look at antidepressants finds low risk of youth suicide. SHOTS: NPR Health Blog. 2012. Feb 7, Retrieved from: http://www.npr.org/blogs/health/2012/02/06/146481573/a-fresh-look-at-antidepressants-finds-low-risk-of-youth-suicide.
- Spielmans GI, Jureidini J, Healy D, Purssey R. Inappropriate data and measures lead to questionable conclusions. JAMA Psychiatry. 2013;70(1):121–122. doi: 10.1001/jamapsychiatry.2013.747. [DOI] [PubMed] [Google Scholar]
- Tsapakis ME, Soldani F, Tondo L, Baldessarini RJ. Efficacy of antidepressants in juvenile depression: Meta-analysis. British Journal of Psychiatry. 2008;193:10–17. doi: 10.1192/bjp.bp.106.031088. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Silva SG, Rohde P, Kratochvil CJ, Kennard BD, Reinecke MA, March JS. Suicidal events in the Treatment for Adolescents With Depression Study (TADS) Journal of Clinical Psychiatry. 2009;70:741–747. doi: 10.4088/jcp.08m04607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Brent D, Greenhill L, Emslie G, Wells K, Walkup J, Zelazny J. Depressive symptoms and clinical status during the Treatment of Adolescent Suicide Attempters (TASA) study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:997–1004. doi: 10.1097/CHI.0b013e3181b5db66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: Systematic review of published versus unpublished data. Lancet. 2004;363:1341–1345. doi: 10.1016/S0140-6736(04)16043-1. [DOI] [PubMed] [Google Scholar]
- Wijlaars LPMM, Nazareth I, Petersen I. Trends in depression and antidepressant prescribing in children and adolescents: A cohort study in the Health Improvement Network (THIN) PLoS ONE. 2012;7(3):e33181. doi: 10.1371/journal.pone.0033181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito J. Rising prevalence of antidepressants among US youths. Pediatrics. 2002;109(5):721–727. doi: 10.1542/peds.109.5.721. [DOI] [PubMed] [Google Scholar]