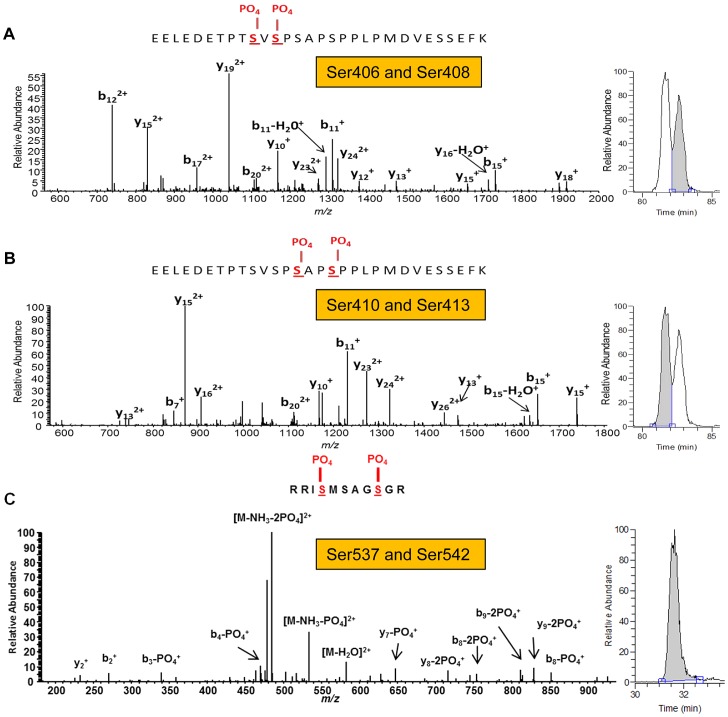
Figure 5. CnaA is phosphorylated at the Serine-Proline Rich Region in vivo.
(A, B) Tandem mass spectra of (A) EELEDETPT[pS]V[pS]PSAPSPPLPMDVESSEFK and (B) EELEDETPTSVSP[pS]AP[pS]PPLPMDVESSEFK from CnaA subunit reveal four unique phosphorylated serine residues (406, 408, 410 and 413) in close proximity. The presence of each identified C-terminal (y) and N-terminal (b) product ions are indicated within the peptide sequence. For additional verification of the unique localization of phosphorylation between the two peptides, corresponding full MS extracted ion chromatograms of m/z 1131.1415 (+/−10 ppm) are shown on the right of each mass spectrum and illustrate a clear chromatographic shift in retention time between the species. (C) Tandem mass spectrum of RI[pS]MSAGSGR from CnaA subunit revealed two unique phosphorylated serine residues (537 and 542). The presence of each identified C-terminal (y) and N-terminal (b) product ions are indicated within the peptide sequence. The single peak in the corresponding full MS extracted ion chromatogram of m/z 591.2272 (+/−10 ppm) shown to the right of the mass spectrum indicates that S537 and S542 were the only two phosphorylated residues within the peptide.