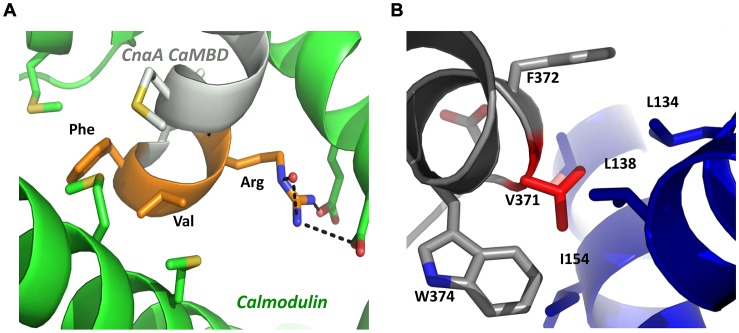
Figure 11. Model of the CaMBD and the CnaB-binding pocket in A.
fumigatus CnaA. (A) The RVF sequence (orange) in the CnaA CaMBD (gray) interacts specifically with calmodulin (green). The arginine residue makes several important salt bridges to aspartic acid residues in calmodulin, which would be lost upon mutation to alanine. The valine and phenylalanine residues bind in a hydrophobic pocket comprising several methionine residues. Mutation of these residues to alanine would result in the loss of important van der Waals interactions and significantly destabilize the calcineurin-calmodulin interaction. (B) V371 lies in a hydrophobic pocket at the interface between CnaA and CnaB subunits. V371D would unfavorably place a charged residue in this pocket.