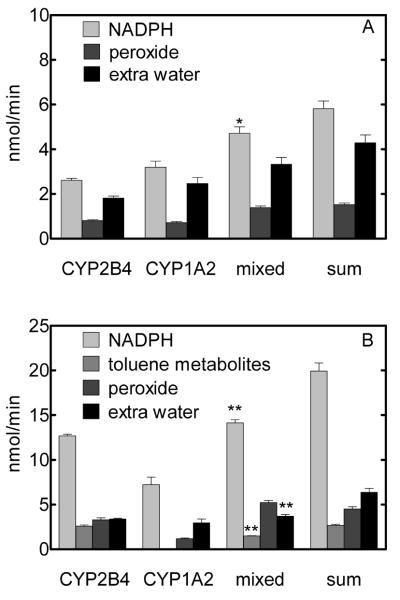
Figure 3.
Effect of CYP1A2•CYP2B4 interactions on P450 reaction coupling. Reconstituted systems of DLPC, CPR, and the P450 enzyme(s) were prepared as described in Materials and Methods. The averages and standard errors (n ≥ 5) of the rates of NADPH consumption and the formation of the products are expressed as nmol/min. The significance of the differences in the rates by the mixed reconstituted system and the sum of those by the simple systems are indicated for each reaction product (*, p < 0.05; **, p < 0.001). Panel A shows the rates of NADPH consumption and the production of hydrogen peroxide and excess water in the absence of substrate. Hydrogen peroxide represents the total amount of hydrogen peroxide and superoxide (which dismutates to hydrogen peroxide and oxygen) formed by decay of the peroxyferrous and oxyferrous intermediates, respectively, of the P450 catalytic cycle (3). Thus, a mole of NADPH is consumed for every mole of hydrogen peroxide formed. Excess water was calculated based on the NADPH consumed and the sum of the various products of these P450-dependent reactions. Extra water is formed by two-electron reduction of the putative, high valent iron-oxo intermediate (iron-oxene species) at the third branch point of the catalytic cycle (6). The iron-oxene species, in turn, is formed following the two-electron reduction of molecular oxygen bound to the heme group of the P450. Thus, the formation of a single molecule of excess water requires the reducing equivalents from two molecules of NADPH. In panel B, toluene metabolites include the sum of the rates of formation of o-cresol, p-cresol, and benzyl alcohol.