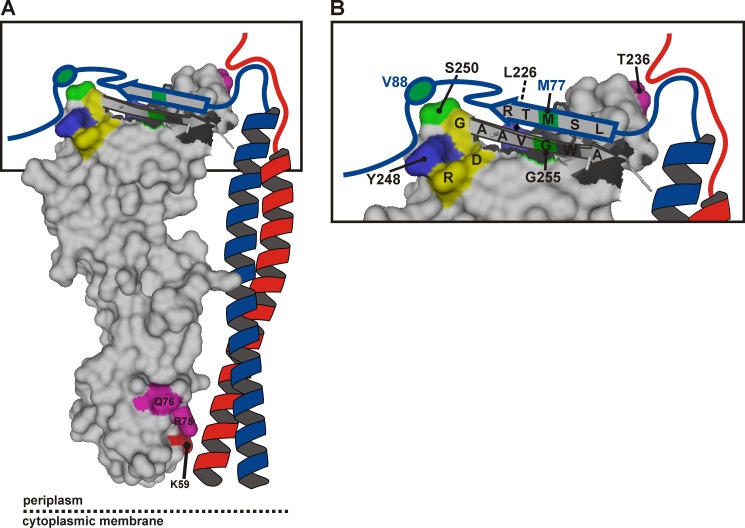
FIGURE 7.
Model of the periplasmic part of the E. coli FtsQBL complex. A, a surface plot of the periplasmic domain of FtsQ exposing the C-terminal β strand in graphic style was created in PyMOL using PDB code 2VH1. The color coding is as in Fig. 1, except for the cysteine cross-linking positions FtsQ 250 and 255 and FtsB 77 and 88, here indicated in green. FtsB (blue) and FtsL (red) are drawn schematically forming a coiled-coil that contacts FtsQ in the α domain around residue 59. B, a close-up of the distal end of the model. The C-terminal regions of FtsB and FtsL are both in close proximity to residue Thr-236 of FtsQ. The C-terminal region of FtsB around residue 77 further engages in a β sheet-like interaction with the C-terminal β strand of FtsQ, whereas the region around residue 88 interacts with the hot spot on FtsQ including residues Leu-226, Tyr-248, and Ser-250. Surrounded by these extensive interactions are residues Asp-245, Arg-247, and Gly-251 (yellow) that are part of a strong consensus motif, DLRY(d/e)(s/t)G.