FIGURE 1.
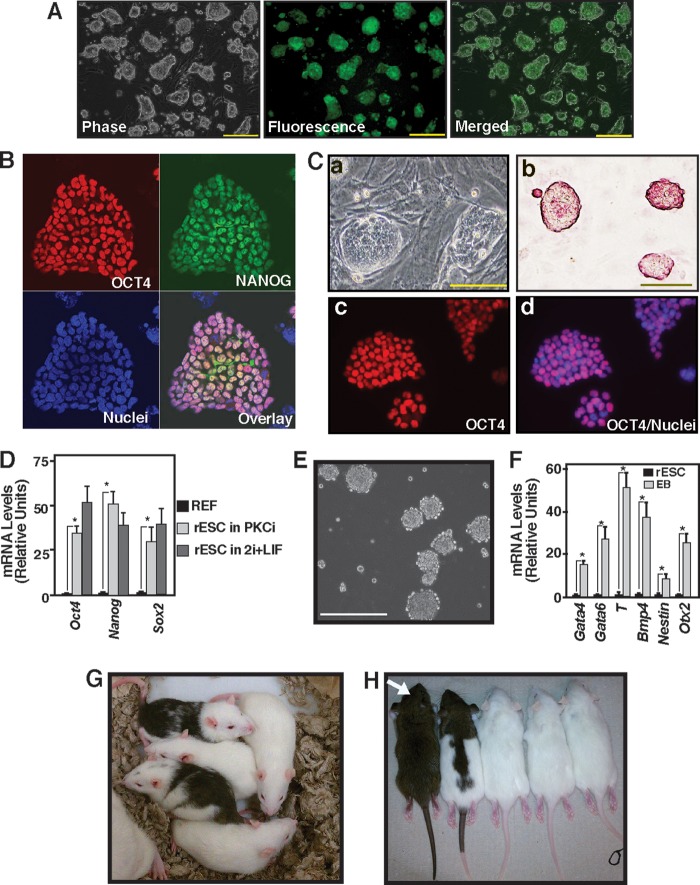
Inhibition of PKC signaling supports rESC self-renewal. A, micrographs of an OCT4-EGFP reporter expressing F344 rESCs showing undifferentiated colony morphology and reporter GFP expression after they were cultured for five consecutive passages with PKCi. Scale bars = 250 μm. B, immunofluorescence images show OCT4 and NANOG expression in F344 rESCs that were maintained with PKCi for seven consecutive passages. C, micrographs showing undifferentiated colony morphology (a), presence of alkaline phosphatase (b), and OCT4 expression in the nuclei (c and d) in DA rESCs after culturing for seven consecutive passages with PKCi. Scale bars = 100 μm. D, plot showing relative mRNA expression of the pluripotency genes Oct4, Nanog, and Sox2 in DA rESCs after they were maintained for seven passages in PKCi and 2i/LIF culture conditions. DA REFs were used as negative controls. Gene expressions were measured by qRT-PCR analyses (mean ± S.E., three independent experiments). *, p ≤ 0.05. E, micrograph showing day 5 EBs that were formed from DA rESCs after PKCi withdrawal. Scale bar = 250 μm. F, differentiation potency in PKCi-maintained DA rESCs was determined by measuring mRNA expression (mean ± S.E., three independent experiments) in day 5 EBs. Undifferentiated DA rESCs were used as a control. The plot shows significant (p ≤ 0.01) induction in lineage-specific markers (Gata4 and Gata6 for endoderm, Bmp4 and T for mesoderm, and Nestin and Otx2 for ectoderm) after a 5-day absence of PKCi and EB formation. G, chimeric rats generated with DA rESCs that were cultured with PKCi. H, germ line offspring (white arrow) from the DA chimeras shown in G.