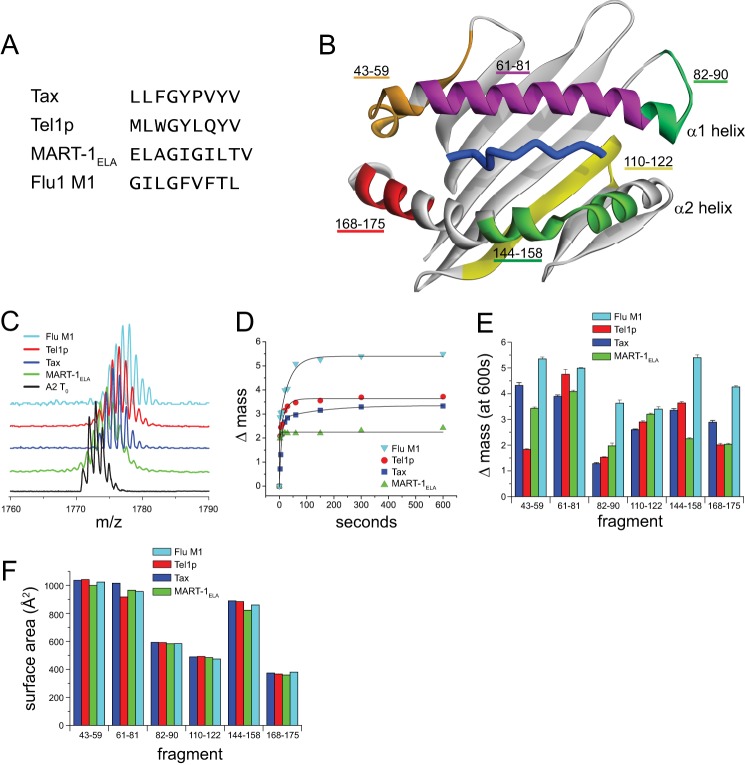
FIGURE 1.
Variation in hydrogen/deuterium exchange properties suggests a peptide dependence to HLA-A2 molecular flexibility. A, sequences of the four peptides whose complexes with HLA-A2 were examined. Each peptide has optimal primary anchors for HLA-A2. B, the different peptidic fragments of the peptide-binding groove identified by HDX-MS mapped onto the HLA-A2 peptide-binding domain. Only residues 1–180 of the HLA-A2 protein are shown. C, mass spectrometric envelopes for the fragment comprising residues 144–158 of the α2 helix at time 0 and after 10 min of exchange for the different peptide·HLA-A2 complexes studied. D, centers of mass for the 144–158 fragment as a function of time out to 10 min of exchange for each of the complexes. Solid lines represent fits to either a single or a double exponential equation. E, mass increase after 10 min of exchange for each of the peptic fragments of the HLA-A2-binding groove in the various complexes. Error bars reflect S.E. at 10 min of exchange from exponential fits as in panel D. F, solvent-accessible surface area of each of the peptide fragments calculated from the crystallographic structures of the different peptide·HLA-A2 complexes. The variation in the structures is small when compared with the variation in hydrogen/deuterium exchange, and there is no correlation between the surface area and isotope exchange datasets.