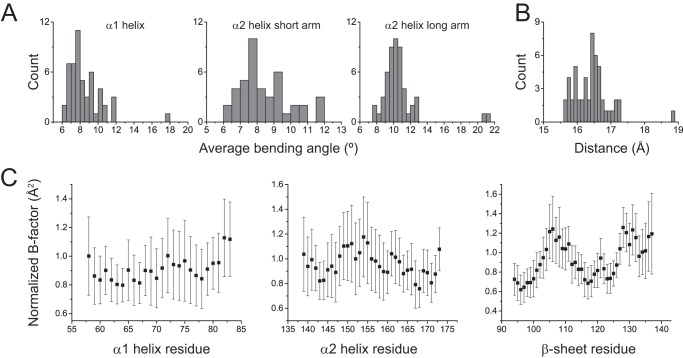
FIGURE 3.
Variation in HLA-A2-binding groove geometry with different peptides. A, distributions of the average bending angles for the α1 helix and the short and long arms of the α2 helix in a database of 51 nonredundant peptide·HLA-A2 structures. B, distribution of the width of the HLA-A2 peptide-binding groove as measured from the α-carbons of Ala-69 and Gln-155 in the 51 peptide·HLA-A2 complexes. C, average normalized B factors in the 51 peptide·HLA-A2 complexes for α-carbons of the α1 helix, α2 helix, and C-terminal half of the β-sheet of the peptide-binding groove. Error bars show the standard deviations, highlighting the considerable variation in B factor with peptide.