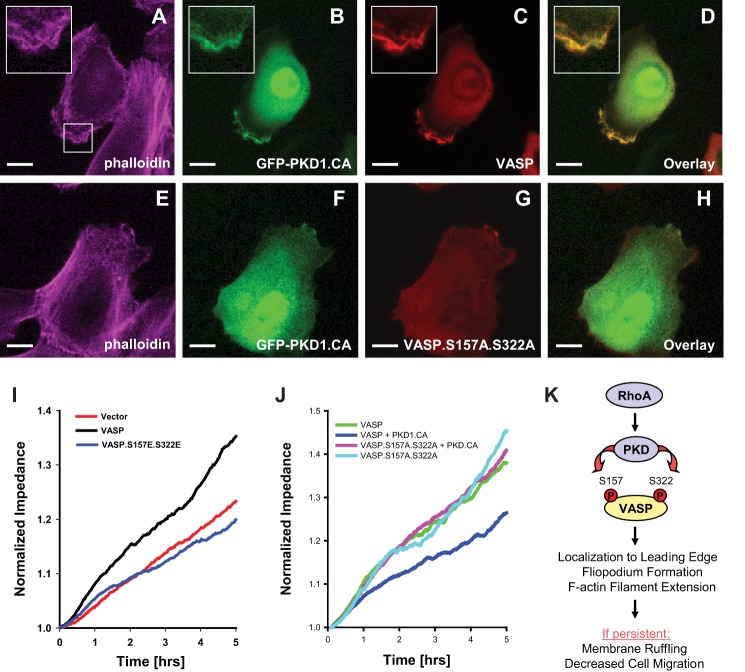
FIGURE 10.
PKD1-mediated VASP phosphorylations induce membrane ruffling and negatively affect cell migration. A–H, HuMEC cells were co-transfected with GFP-tagged active PKD1 (GFP-PKD1.CA) and VASP, or the VASP.S157A.S322A mutant as indicated. 16 h after transfection, cells were fixed, and F-actin was stained with phalloidin. Localization of proteins was determined using immunofluorescence analysis (bar is 10 μm). Insets are 2.5-fold enhanced. Changes in cellular localization were observed in all cells (typically n = 50) analyzed. All data were confirmed in at least three independent experiments. I and J, HeLa cells were transfected with vector control, VASP or VASP phosphorylation mimicking mutant as indicated (I), or with vector or PKD1.CA in combination with VASP or the VASP.S157A.S322A mutant (J). Real-time cell migration was monitored with an impedance-based assay system (ECIS) over a time period of 5 h. K, schematic of the here proposed PKD1-mediated signaling events downstream active RhoA that lead to VASP localization to the leading edge of cells, to filopodia formation and cause F-actin filament extension. If persistent such signaling results in membrane ruffling and eventually to decreased cell migration.