FIGURE 6.
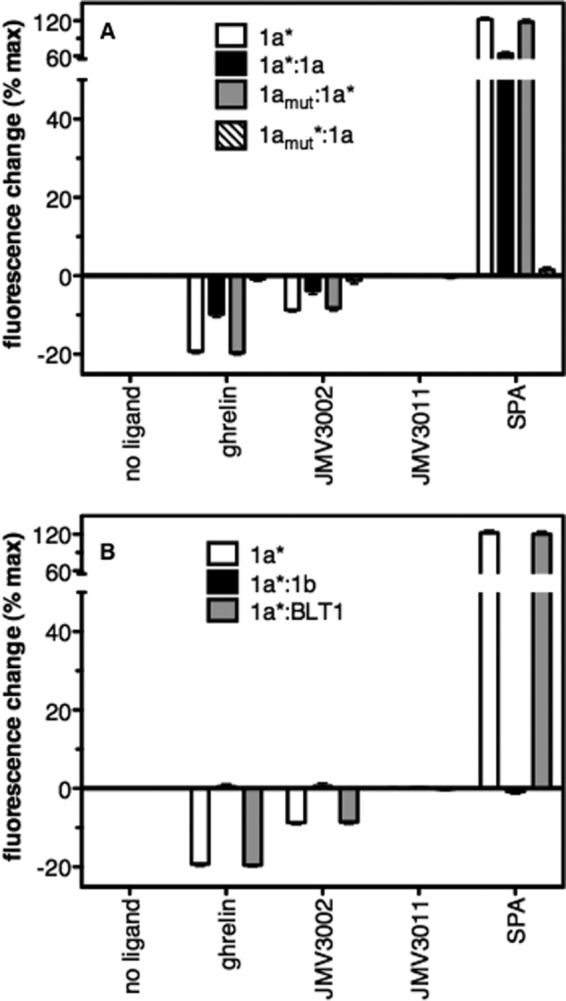
Influence of dimerization on ligand-induced GHS-R1a conformational changes. Shown are the changes in maximum emission intensity of bimane attached to GHS-R1a in the absence and presence of ligands (full agonist, ghrelin; partial agonist, JMV3002; neutral antagonist, JMV3011; inverse agonist, SPA; ligand concentration, 10 μm). Data are presented as percent changes in maximum emission intensity compared with that of the monomeric receptor in the absence of ligand. 1a, GHS-R1a monomer; 1a:1a, GHS-R1a-GHS-R1a homodimer; 1amut:1a, GHS-R1aE124Q-GHS-R1a dimer; 1a:1b, GHS-R1a-GHS-R1b dimer; 1a:BLT1, GHS-R1a-BLT1 dimer. The asterisk indicates the protomer labeled with bimane. In all cases, data represent the mean ± S.D. from three independent experiments.
