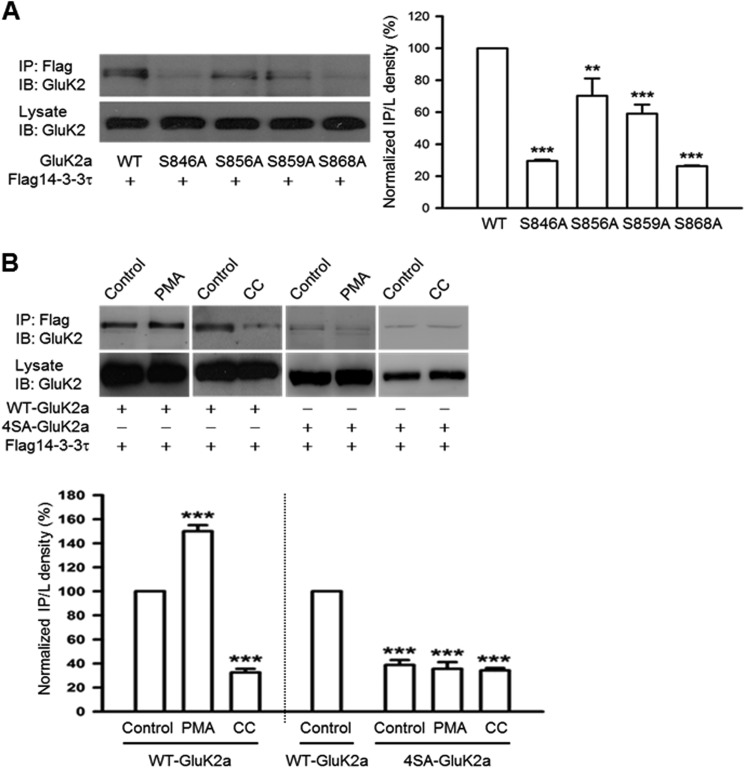
FIGURE 2.
14-3-3 proteins bind to the C terminus of GluK2a in a PKC phosphorylation-dependent manner. Western blot analyses of immunoprecipitates and cell lysates from HEK293 cells were transfected with 14-3-3τ and other proteins as indicated. A, mutation of single serine residues at the GluK2a C terminus impairs interaction between 14-3-3 and GluK2a. Quantification of Western blots indicates that the S846A and S868A mutants have more significant reduction in 14-3-3 binding (n = 3, **, p < 0.01; ***, p < 0.001, comparison with WT) (right panel). B, coimmunoprecipitation of 14-3-3 and WT GluK2a is enhanced by PMA (5 μm) (a PKC activator) and reduced by calphostin C (1 μm) (a PKC inhibitor, CC). Right panel shows that the mutant 4SA-GluK2a subunit has impaired 14-3-3 binding, which is not affected by either PMA or calphostin C treatment. Lower panel summarizes quantified Western blot results for groups data of PMA (n = 4), CC (n = 3) in WT GluK2a, and PMA (n = 3), CC (n = 3) in 4SA-GluK2a. Data represent mean ± S.E., ***, p < 0.001, comparison with WT control. IB, immunoblot; IP, immunoprecipitation.