FIGURE 6.
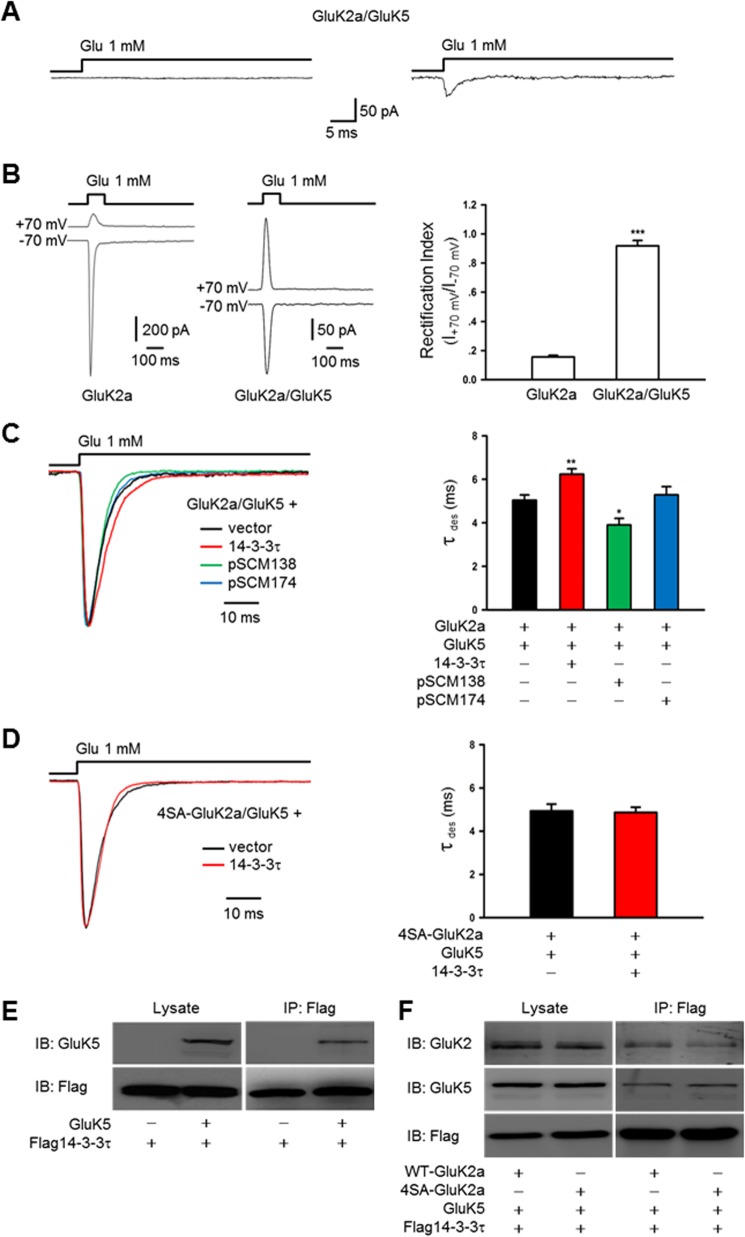
14-3-3 proteins modulate the kinetics of heteromeric GluK2a-containing receptors in a heterologous system. A, GluK2a/GluK5 currents recorded in outside-out configuration from cotransfected HEK293 cells. In our experimental conditions, most of the patches showed either no detectable current (left panel, n = 20) or small currents ranging from 30 to 50 pA (right panel, n = 3). B, representative whole-cell currents recorded from HEK293 cells transfected with GluK2a, alone or together with GluK5 subunits. GluK2a and GluK5 cDNAs were transfected at a ratio of 1:3. Glutamate pulses (1 mm, 100 ms) delivered to cells expressing homomeric GluK2 subunits elicited current responses of large amplitude at a holding potential of −70 mV, whereas the peak current amplitude was considerably smaller in GluK2a/GluK5-cotransfected cells (left panel). The rectification index (ratio between the current amplitudes at membrane potential +70 mV and −70 mV) of currents recorded from cells transfected with GluK2a alone or together with GluK5. Quantification data show means ± S.E. of GluK2a (n = 9) and GluK2a/GluK5 (n = 11) groups. ***, p < 0.001 (right panel). For recordings from GluK2a/GluK5-cotransfected cells, data with rectification index of <0.9 were discarded. C, representative whole-cell current traces from HEK293 cells cotransfected with GluK2a and GluK5 subunits, in combination with other proteins as indicated. Desensitization kinetics of heteromeric GluK2a/GluK5 receptors is also slowed by exogenous 14-3-3τ (red trace) and accelerated by cotransfection of pSCM138 (green trace) but not pSCM174 (blue trace). Right panel summarizes group data for vector- (n = 14), 14-3-3τ- (n = 28), pSCM138- (n = 8), and pSCM174 (n = 8)-cotransfected cells. Data represent mean ± S.E.; statistical significance is denoted as follows: *, p < 0.05; **, p < 0.01. D, representative whole-cell current traces from HEK293 cells cotransfected with 4SA-GluK2a mutant and GluK5 in combination without or with 14-3-3τ. Cotransfection of 14-3-3τ (red trace) has no effect on the desensitization of heteromeric 4SA-GluK2a/GluK5 receptors. Right panel shows the summary data (n = 12 and 13 for without and with 14-3-3τ, respectively). E and F, 14-3-3τ associates with GluK5 subunits. Western blot analyses of immunoprecipitates (IP) and cell lysates from HEK293 cells transfected with 14-3-3τ and other proteins as indicated. E, 14-3-3τ and GluK5 subunits coimmunoprecipitate in cotransfected cells. F, interaction between 14-3-3τ and GluK5 subunits is not dependent on 14-3-3 binding to the GluK2a subunit. The results shown are representative of at least three independent experiments. IB, immunoblot.