FIGURE 4.
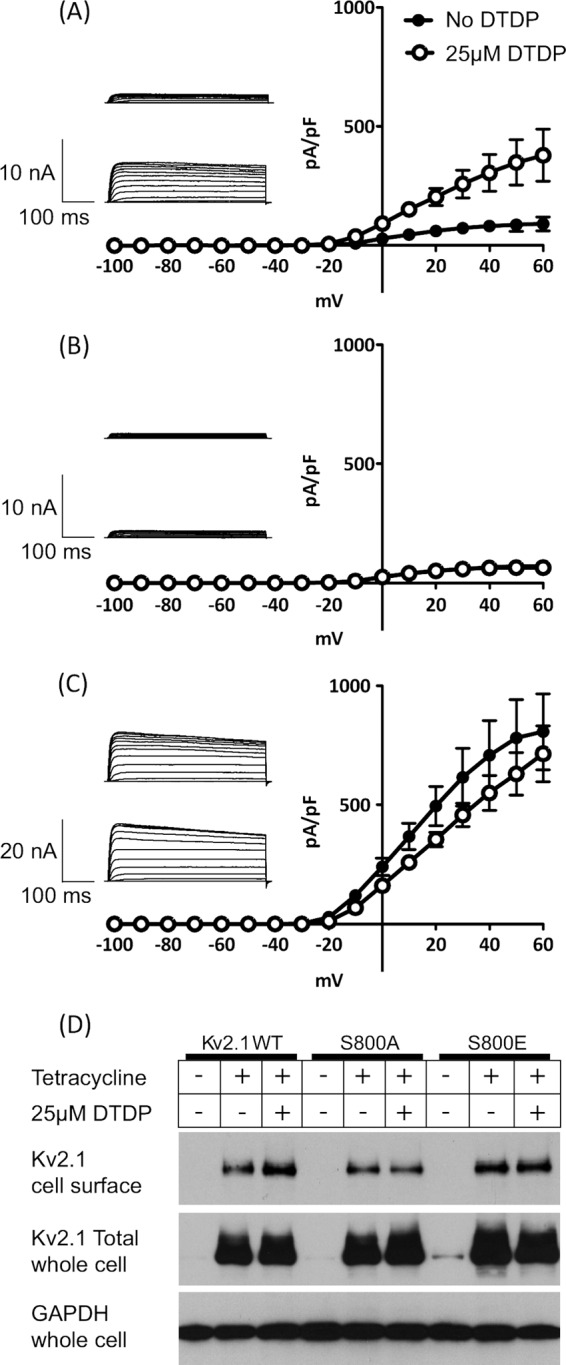
Kv2.1 ion channel activity is regulated by serine 800 phosphorylation. Whole-cell patch clamp recordings of Kv2.1 K+ currents. HEK293 cell lines expressing wild type Kv2.1 (A), S800A (non-phosphorylatable mutant: B) or S800E (phosphomimetic mutant: C) were subjected to whole cell patch clamp in the presence (○) or absence (●) of the oxidant DTDP (n = 3). Insets show representative traces of K+ currents in patch-clamp recordings obtained by step depolarizations applied from a holding potential of −70 mV to between −100 mV and +60 mV, in 10-mV increments. Top; trace without DTDP stimulation, below: with DTDP stimulation (D) HEK293 Kv2.1 cells were incubated with tetracycline to induce Kv2.1 expression, and/or DTDP to induce oxidative stress. Proteins expressed on the plasma membrane of HEK293 Kv2.1 cells were biotin-labeled and harvested samples were probed by Western blot with anti-Kv2.1 antibody (top panel). Whole cell lysates were also probed with the total Kv2.1 (middle). Comparable amount of Kv2.1 proteins were expressed following addition of tetracycline. GAPDH detection was performed as a control (bottom).
