FIGURE 7.
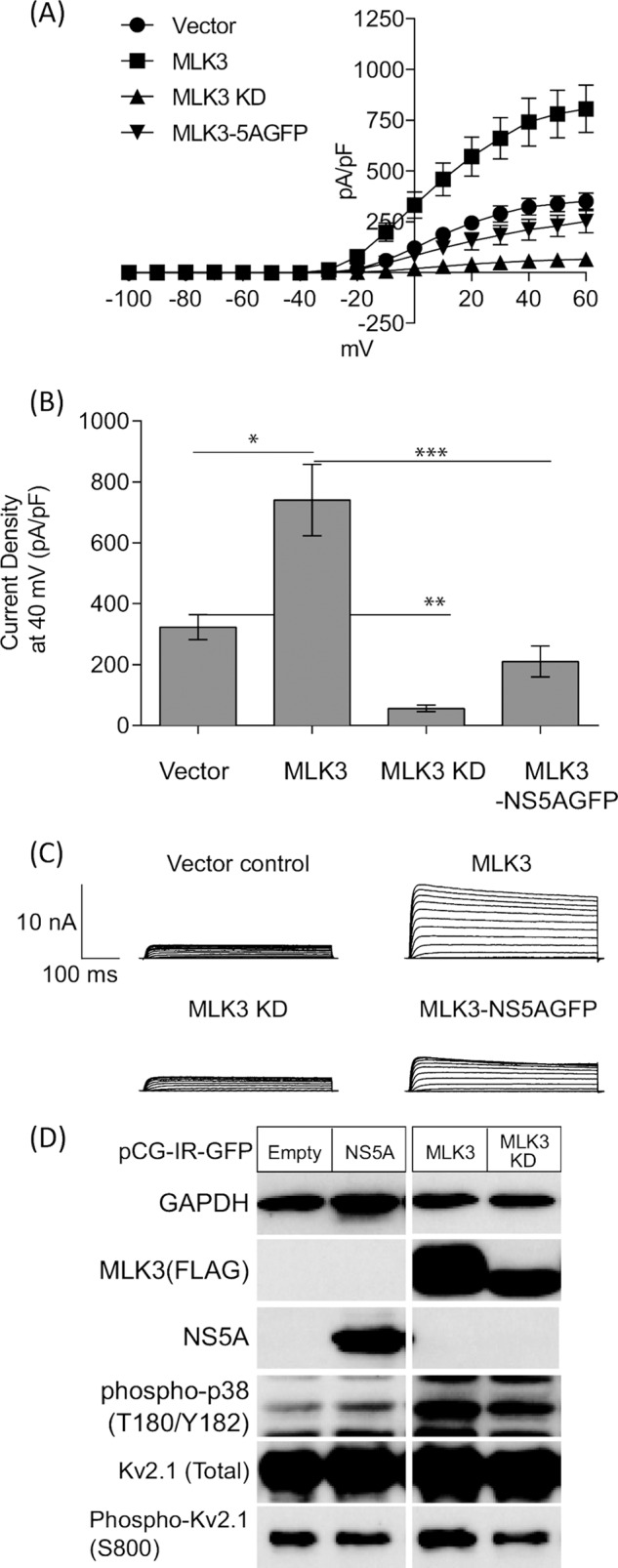
MLK3 acts as an upstream regulator of Kv2. 1 channel via Ser800 phosphorylation. A, HEK293 cells expressing Kv2.1 were transfected with bicistronic vectors expressing MLK3 (wild type or K144E) together with GFP or NS5A-GFP as shown in Fig. 1B. 16 h post-transfection, Kv2.1 expression was induced by tetracycline treatment, and 8 h later GFP-positive cells were identified by fluorescent microscopy and subjected to electrophysiological recordings. Overexpression of MLK3 wild type augments Kv2.1 channel activity (■), whereas, the K144E kinase-inactive mutant suppressed channel activity (▴). Co-expression of NS5A-GFP abrogated the MLK3 mediated enhancement of Kv2.1 activity (▾). B, comparison of current density measurements at +40 mV. *, p < 0.02, **, p < 0.001, and ***, p < 0.005, unpaired t test. C, representative traces of outward K+ currents in patch-clamp recordings. D, lysates from HEK293 cells expressing Kv2.1 wild type were analyzed by Western blotting with the indicated antibodies. Cells were transfected as follows: lane 1: empty vector, lane 2: NS5A-IRES-GFP, lane 3: MLK3 wild type-IRES-GFP, and lane 4: MLK3 K144E-IRES-GFP.
