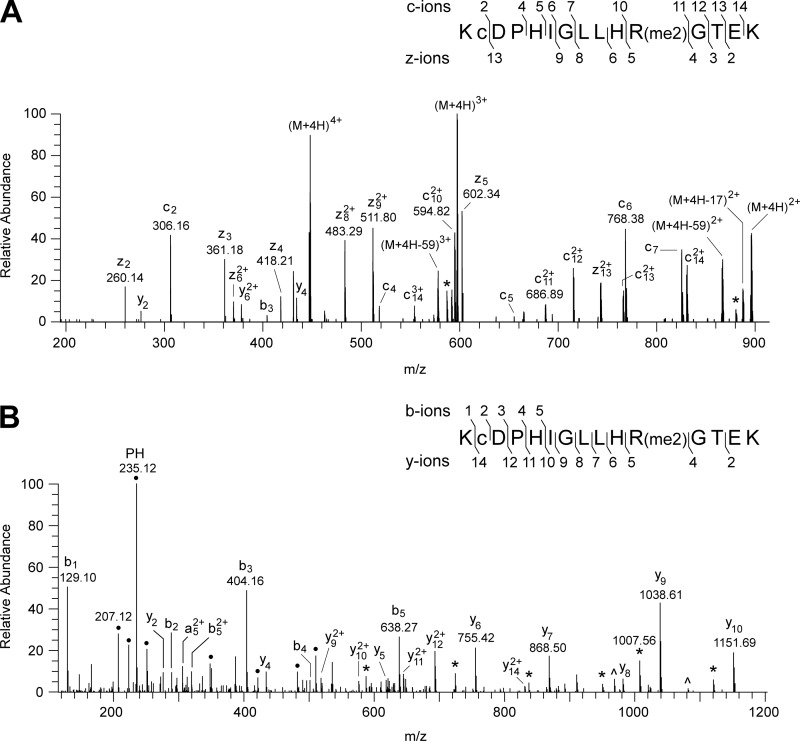
FIGURE 3.
Characterization of the symmetrical dimethylation of residue Arg-85 of the 49-kDa subunit of bovine complex I. A and B, spectra of fragments produced, respectively, by ETD of a quadruply charged ion (447.99 m/z) and by HCD of a triply charged ion (596.99 m/z), both derived from the tryptic peptide corresponding to residues 75–89 of the 49-kDa subunit. In A, a singly charged ion (447.12 m/z) is a contaminant of the precursor (M+4H)4+ ion. In B, ● denotes ions arising by internal fragmentation. The ions z4-z5, c10-c11, and y4-y5 demonstrate dimethylation of residue Arg-85 of the 49-kDa subunit. The losses of the neutral fragments with masses of 31 (monomethylamine) from y5-y10 and 70 (dimethylcarbodiimide) from y9 and y10, labeled * and ∧ respectively, define the modification as the symmetrical dimethylation of the guanidino group of residue Arg-85. In the insets, the fragment ions are mapped onto the amino acid sequence; c is carbamidomethylcysteine.