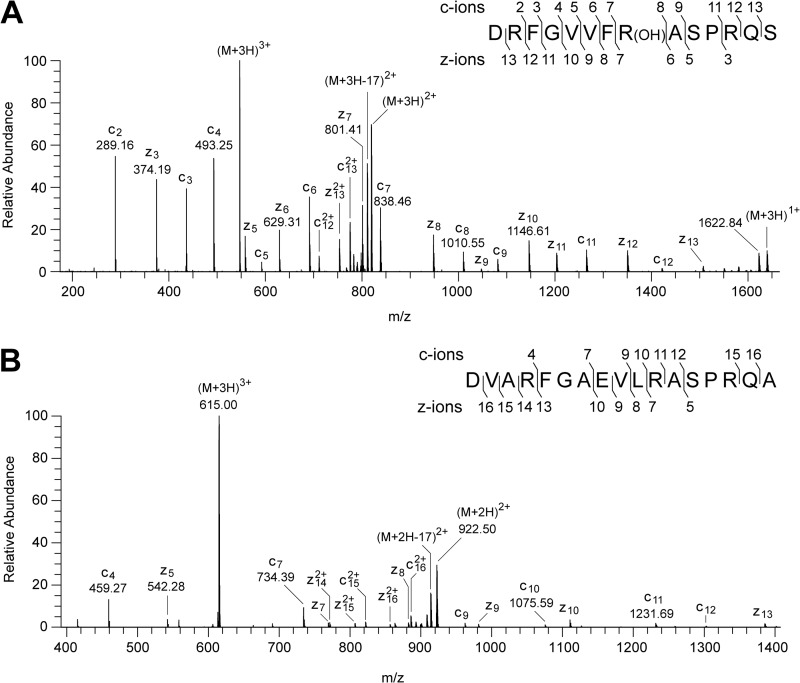
FIGURE 8.
Characterization of the hydroxylation of residue Arg-73 of the PSST subunit of human complex I and the unmodified residue in the E. coli NuoB subunit. A, ETD fragmentation spectrum of a triply charged ion, m/z 546.62, generated by cleavage of the human PSST protein with Asp-N. The ions z6-z7 and c7-c8 show that the +16 Da modification is associated with residue Arg-73. B, spectrum of fragments produced by ETD from a triply charged ion (m/z 615.00) from a peptide corresponding to residues 77–93 of the E. coli NuoB subunit. The series of fragment ions identifies the peptide and excludes modification of residue Arg-87. In the insets, the fragment ions are mapped onto the amino acid sequence.