Fig. 3.
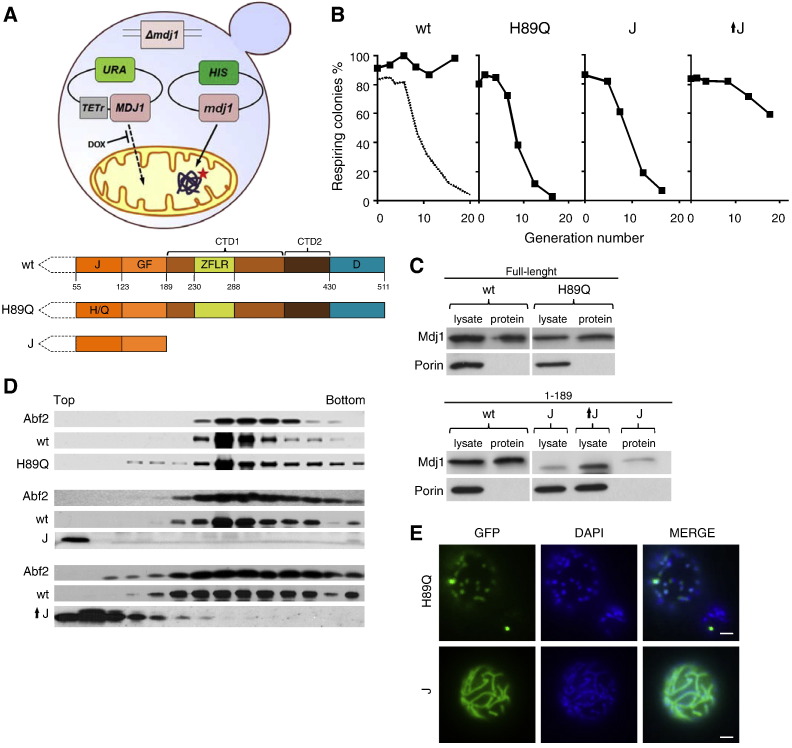
Functional J-domain of Mdj1 is critical for maintenance of mtDNA. A) (Top) Scheme of yeast strain used to analyze the effect of Mdj1 variants on the maintenance of mtDNA upon depletion of wt Mdj1. mdj1-Δ [TETr-MDJ1] cells were transformed with a plasmid harboring a mutant mdj1 gene under control of the native MDJ1 promoter. Since the wt MDJ1 gene is under the control of the TETr promoter, addition of doxycycline (DOX), which represses expression, allows testing of the effect of mutations upon depletion of the wt protein. (Bottom) Scheme of domain composition of Mdj1 variants. N-terminal mitochondrial presequence (dashed line). J-module consists of J-domain (orange) followed by glycine/phenylalanine (GF) -rich linker region (light orange). C-module includes C-Terminal Domain 1 (CTD1; light brown) with extruding Zinc Finger Like Region (ZFLR; green) and C-Terminal Domain 2 (CTD2; brown). D-module is the putative dimerization domain (blue). B) Yeast cells (described in A) expressing the MDJ1 proteins: as a control, wild-type (wt), the J-domain variant Mdj1H89Q (H89Q) or the J-domain containing fragment Mdj1Δ190–511 (J); or overexpressing Mdj1Δ190–511 from the TEF promoter (↑J) were grown on glucose-based media in the presence of doxycycline. At the indicated number of generations after doxycycline addition aliquots were collected and plated on glucose-based media. The percentage of respiring cells was taken to be the ratio of the number of red colored colonies to the total number of colonies. For comparison, the data from Fig. 2C in which a second plasmid expressing Mdj1 is absent is indicated by a dashed line in the wt plot and thus represents the loss of respiratory competence upon depletion of Mdj1. C) Determination of relative levels of wt Mdj1 and Mdj1 variants in mitochondria. Mitochondrial lysates prepared from cells after growth in the presence of doxycycline for 10 generations, were separated by electrophoresis and subjected to immunoblot analysis using antibodies specific for Mdj1 and, as loading control, porin. Because the polyclonal antibody used reacts differently with wt and truncated forms of Mdj1, 0.2 pmol of purified wt Mdj1 or indicated variants was run on the same gel (protein), as a semi-quantitative reference for expression. Slower migration of the purified J variant is due to the presence of 6 His tag at the C-terminus. D) Distribution of Mdj1 variants after centrifugation of mitochondrial lysates. Mitochondrial lysates isolated from MDJ1/mdj1-Δ diploid cells expressing the Mdj1 variants Mdj1H89Q (H89Q) and (J) under control of native MDJ1 promoter or overexpressed (↑J) under control of TEF promoter, grown in glucose-based medium were subjected to ultracentrifugation through a sucrose gradient. Each fraction was analyzed for protein content using immunoblot analysis with antibodies specific for Mdj1 or, as a control, Abf2. In the case of Mdj1H89Q, a fusion with GFP was used to allow separation from wt Mdj1 during electrophoresis. E) Cellular localization of Mdj1 variants. MDJ1/mdj1-Δ diploid cells constitutively expressing GFP fusions of Mdj1H89Q (H89Q) or Mdj1Δ190–511 (J) were analyzed by fluorescence microscopy. Cellular DNA was stained with DAPI. Overlay of the two images (MERGE). Size bars (2 μm) are shown.