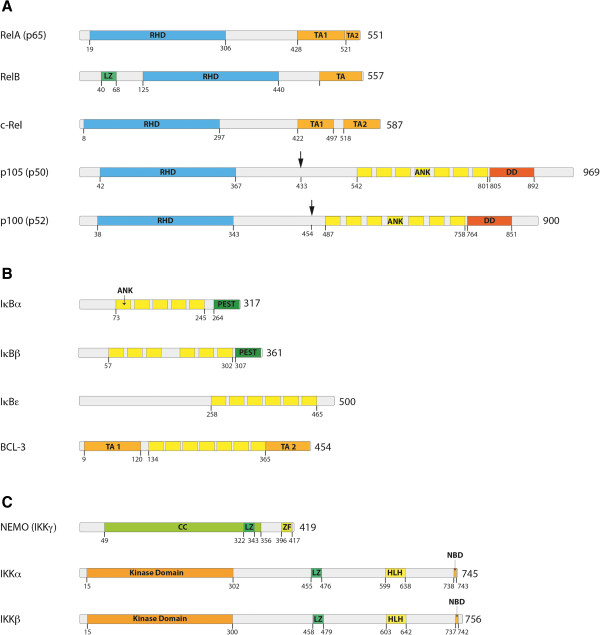
Figure 1.
Members of the NF-κB signaling pathway and the IκB kinase-complex. (A) The five members of the NF-κB family of proteins: RelA (p65), RelB, c-Rel,NF-κB1 (p105), and NF-κB2 (p100). p105 and p100 are processed to their shorter forms p50 and p52, respectively. All members of the NF-κB family harbor an N-terminal Rel homology domain (RHD), which mediates DNA contact and homo- and heterodimerization. Three family members (RelA, RelB and c-Rel) contain C-terminal transactivation domains (TAs), which are essential for transcriptional activity. (B) The IκB family of proteins consists of four members: IκBα, IκBβ, IκBϵ and BCL-3. These proteins are characterized by the presence of ankyrin (ANK) repeats, which mediate binding of IκBs to the NF-κB family of proteins. Based on the presence of ankyrin repeats, p100 and p105 can also be included into the IκB family – as their DNA-binding RHD domain is covalently linked to an IκB-like inhibitory domain. In addition to the ANK repeats IκBα and IκBβ contain PEST domains, which are enriched in proline, glutamate, serine and threonine and are required for constitutive turnover. BCL-3 differs from other IκB family members by containing TA domains, which mediate transcriptional activity when BCL-3 is associated with NF-κB dimers that bind to DNA. (C) The three most important members of IκB kinase (IKK) complex: NF-κB Essential Modulator (NEMO or IKKγ), IκB kinase α, (IKKα or IKK1) and IκB kinase β (IKKβ or IKK2). Further abbreviations: leucin-zipper-like motif (LZ), death domain (DD), coiled-coil domain (CC), zinc-finger domain (ZF), helix-loop-helix domain (HLH), NEMO-binding domain (NBD). It is important to note that the total number of amino acids of protein as well as the start and end of some domains can differ between publications and databases.