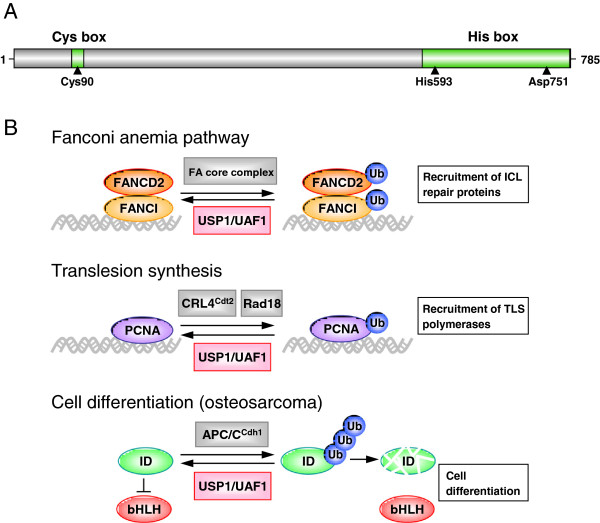
Figure 1.
USP1 Domain structure and summary of the cellular functions of the USP1/UAF1 complex. A. Shematic representation of USP1 protein showing the position of its amino-terminal Cys box and carboxy-terminal His box domains. The catalytic residues Cys90, His593 and Asp751 are indicated by arrowheads. B. Models illustrating the role of the USP1/UAF1 complex as regulator of three different cellular processes. In the Fanconi anemia (FA) pathway (upper panel), the FA core complex monoubiquitinates FANCD2 and FANCI, which mediate the recruitment of other proteins that repair DNA interstrand crosslink (ICL) lesions. USP1/UAF1 antagonizes the FA core complex deubiquitinating FANCD2 and FANCI. In the process of translesion synthesis (TLS) (middle panel), monoubiquitinated PCNA recruits specific TLS DNA polymerases that may bypass DNA lesions, but have lower fidelity than replicative polymerases. USP1/UAF1 may prevent unscheduled recruitment of low fidelity TLS polymerases by deubiquitinating PCNA. Finally (lower panel), USP1/UAF1 contributes to the maintaince of the undifferentiated state of osteosarcoma cells by promoting deubiquitination and stability of ID proteins, which, in turn, negatively regulate differentiation-inducing bHLH transcription factors.