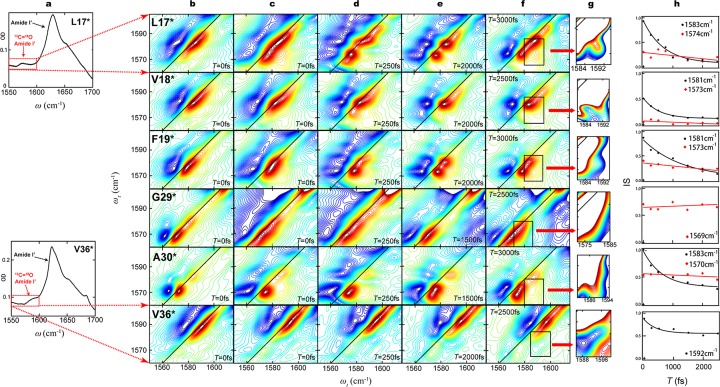
Figure 3.
Spectroscopic results from fibrillar Aβ peptides with 13C=18O labels in 6 different residues. (a) Amide I′ region of FTIR spectra from L17* (above) and V36* (below). The dominant feature at 1625 cm–1 in both spectra arises from peptide groups containing 12C=16O. Peptide groups containing 13C=18O labels yield subtle features in regions outlined by red rectangles. (b) 2D-IR spectra with zero waiting time (T = 0) from amyloid fibrils formed after 10 weeks. Aliquots were withdrawn from solutions of Aβ40 monomers with 13C=18O labels in the six different residues indicated, and dried on CaF2 windows. The vertical axis is the pump frequency (ωτ); the horizontal axis is the probe frequency (ωt). The pump frequencies span the range indicated by red rectangles in column (a). Red contours (positive) represent the 0 to 1 transition of the amide I′ vibration, while blue contours (negative) represent the 1 to 2 transition. The difference in ωt between the positive and negative peaks is due to anharmonicity of the vibrational transition energies. Diagonal lines are the locus of points where ωτ = ωt. The six spectra in this column have been previously published, and are reproduced here to facilitate comparisons across each row. The spectra in each row are from fibrils with labels in the same residue. (c) 2D-IR spectra with zero waiting time (T = 0) from amyloid fibrils formed after 4 years. The samples examined were prepared by withdrawing fresh aliquots from the same solutions that yielded the spectra in column (b) and drying them on CaF2 windows. The spectra shown are representative of those collected from two or more solutions. (d–f) 2D-IR spectra with selected waiting times between 250 and 3000 fs, collected from the same preparations yielding the spectra in column (c). Changes in band shape at various waiting times reflect fast dynamics near the 13C=18O labeled residue. (g) 2D-IR spectra with expanded scales, corresponding to the regions in column (f) outlined in black rectangles. The expanded scales aid in the identification of crosspeaks between absorption bands, which are relatively low in intensity; regions of high intensity absorption are off-scale and are rendered white. (h) Changes in the inverse slope of absorption band nodal line (IS) are plotted as a function of waiting time (T), and fitted to exponentially decaying frequency-frequency correlation functions. Decay constants are listed in Table 1. All 2D spectra are plotted with 60 evenly spaced contour lines with the maximum intensity of each spectrum normalized to 1. The relative intensities of different transitions are shown with different colors from red (positive) to blue (negative).