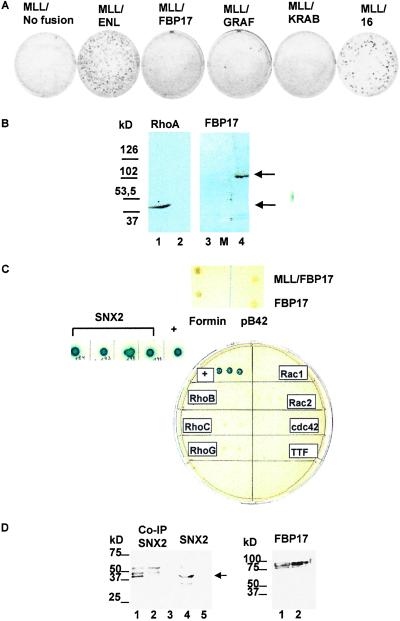
Figure 4.
(A) Results of the in vitro transformation/CFC test after three rounds of replating. Colonies generated per 10,000 input cells. Strong in vitro transformation activity of MLL/ENL and the fusion of MLL with the ENL transactivation domain (MLL/16). Only very few colonies are seen after retroviral transduction with MLL/FBP17. The MLL/GRAF fusion, generated by a recently described (11) translocation t(5;11)(q31;q23), was tested as well. A truncated form of MLL (no fusion) and a fusion of MLL to the KRAB repressor domain revealed no transforming activity. (B) Western blotting of yeast EGY48 cells with anti-RhoA-moAB and polyclonal anti-FBP17 antiserum. Lane 1, RhoA-fusion 44 kDa; lanes 2 and 3, negative controls with preimmunserum; lane M, molecular mass standard (Sigma); lane 4, FBP17-LexA 109 kDa. (C) Results of the two-hybrid screening. None of the Rho family members tested showed an interaction with FBP17. In addition, library screening revealed a specific interaction between SNX2 and FBP17 (blue-colored colonies). Neither FBP17 nor MLL/FBP17 interacted with mouse formin. Positive control shows the interaction between pLexA-p53 and pB42AD large T antigen. All colonies were plated on drop-out medium, without tryptophane, uracil, histidine, and leucine (D) Coimmunoprecipitation of FBP17 and SNX2. (Left) Lane 1, GFP-SNX2 after coprecipitation with myc-FBP17. Note the strong signal at 42 kDa. The detection was performed with an anti-myc antibody. The 42-kDa GFP-SNX2 protein was detected with an anti-GFP antibody by Western blotting (lane 4). In the control reaction, no coprecipitated SNX2 could be detected (lanes 2, 3, and 5). (Right) The myc-tagged FBP17 protein is expressed in similar amounts in the 293T cells cotransfected with GFP-SNX2 (lane 1) as well as in the negative control transfected only with myc-FBP17 (lane 2).